4789
Quantification of in-vivo myocardial stiffness in the rat heart using transient mechanical waves
Marco Barbero Mota1, Giacomo Annio1, Guillaume Rucher2, Anna Wittgestein3, David Nordsletten3,4, Jordi Martorell5, and Ralph Sinkus1,3
1LVTS U1148, LVTS U1148 INSERM - Université de Paris, Paris, France, 2FRIM, INSERM - Université de Paris, Paris, France, 3School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Biomedical Engineering and Cardiac Surgery, University of Michigan, Ann Arbour, MI, United States, 5Chemical Engineering, IQS, Barcelona, Spain
1LVTS U1148, LVTS U1148 INSERM - Université de Paris, Paris, France, 2FRIM, INSERM - Université de Paris, Paris, France, 3School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Biomedical Engineering and Cardiac Surgery, University of Michigan, Ann Arbour, MI, United States, 5Chemical Engineering, IQS, Barcelona, Spain
Synopsis
Heart biomechanics play a crucial role in the diagnosis and prognosis of cardiac diseases. However, current gold standard methods are highly invasive and preclude continuous monitoring of patients’ myocardium stiffness as a biomarker. In this study, we report the first results of a novel magnetic resonance elastography technique that provides with high temporal and spatial resolution enabling accurate transient shear wave speed values quantification, as thereof travel through heart tissue. Although biased due to the thin-plate geometry of the heart, early and mid-late systole, and early diastole preliminary results intuitively match the expected dynamical biomechanics of the human heart.
Introduction
Magnetic Resonance Elastography is a MRI-based technique that enables non-invasive biomechanics quantification by measuring shear wave propagation in the tissue of interest. In this study a 7T preclinical MRI system was used to investigate the possibility to track the spatio-temporal propagation of externally generated shear waves through the septum of an anesthetized rat, and in so be able to quantify myocardium stiffness “in vivo” throughout the cardiac cycle.Materials and methods
A 3D printed custom-build setup allowed to generate mechanical excitation via a piston located just underneath the thorax. An ECG+respiratory gated 3D GRE Flash CINE sequence (TE=2.3, TR=13ms) was rendered motion sensitive via a bipolar gradient (2ms length, 150mT/m strength) with 11 movie frames covering the whole cardiac cycle of the rat. The sequence was equipped with 1st order gradient moment nulling, and flow compensated prepulse to drastically reduce blood flow related artifacts. The external 166.67 Hz mechanical excitations were delayed by 15, 52 and 104 ms relative to the beginning of the R-R cycle to probe the septum during early systole (ES), mid-late systole (MS) and early diastole (ED), respectively, expecting significant differences in results among the three timepoints.To enhance the otherwise limited temporal resolution of one TR (i.e. 13 ms), image acquisition was repeated 100 times with piston punches detained each repetition by an accumulative delay of 13ms (TR)/100=0.13ms avoiding overlapping with the subsequent movie frame. Image acquisition was locked to the R-R cycle and retrospective re-ordering of the data allowed for transient propagation investigation with artificially higher temporal resolution of 0.13 ms (Figure 1). Image resolution was 1x1x5mm3 and was positioned as an axial slice intersecting the left and right ventricles at their maximum short heart axis radii.
Wave propagation was quantified via waterfall diagrams on unwrapped phase data along predefined lines through the septum at the probed cardiac states. To enhance shear wave signal within the waterfall diagrams we apply two corrections to the phase images one in space and one in time (Figure 2). For the former, the collection of an additional repetition identical to its precedent (100th) but this time without mechanical excitation (101st), enabled to obtain a spatial map of the main magnet heterogeneities across the FOV which was subtracted from all of our acquisitions.For the latter, the positioning of an ultrasound gel filled vial within the FOV enabled us to track the phase shift coming from the temporal drift of the magnet at every acquisition timepoint. Hence, by removing this offset we were capable to correct for this temporal baseline variability when reordering all the repetitions acquisitions.
For the speed quantifications, the time signal at the septum closest point to the piston was taken as reference for temporal cross-correlation along the myocardium tissue (Figure 3). This analysis allows for delay estimation at each image pixel spatial point and consequent speed propagation.
Results and discussion
Preliminary average speed values along error on the mean estimations from 2 specimens (reproducibility) scanned at subsequent repetitions (repeatability) are hereby reported (Figure 4). These reported values are biased due to the waveguide effect of the thin geometry of the septum. However, this geometrical confounder can be estimated via finite element method simulations and corrected for as illustrated by Troelstra et al.1 After correction real speed values can be used to accurately determine shear stiffness of the septum at each probed cardiac cycle timepoint (ES, MS and ED) through viscoelasticity theory. As higher velocity correlate with stiffer tissue, our biased preliminary results give good intuition of matching with the expected myocardium biomechanics of the contraction state at each cardiac cycle phase. Yet exact stiffness values require of bias removal as part of future work.The overarching value of this unprecedented technique resides on the versatility of probing the “in vivo” biomechanics of cardiac tissue at any timepoint during the cardiac cycle with high temporal and spatial
Acknowledgements
No acknowledgement found.References
[1] Troelstra, M.A., Runge, J.H., Burnhope, E. et al. Shear wave cardiovascular MR elastography using intrinsic cardiac motion for transducer-free non-invasive evaluation of myocardial shear wave velocity. Sci Rep 11, 1403 (2021). https://doi.org/10.1038/s41598-020-79231-zFigures
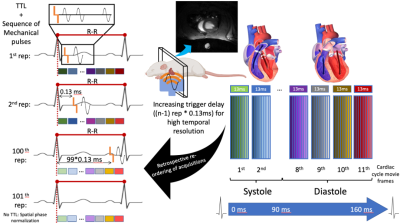
Figure 1. Diagram summarizing the temporal transient MRE image acquisition and acquisition re-ordering workflow. TTL: transistor-transistor logic pulse (trigger); n = 100, number of TTL repetitions.
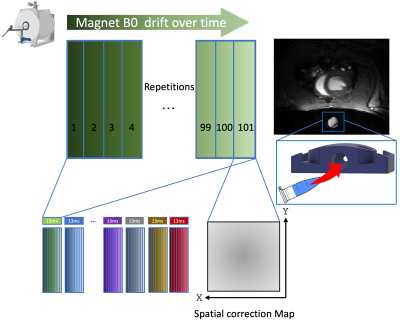
Figure 2. Waterfall diagrams phase corrections. Spatial phase correction is derived from the 101st image acquisition of each experiment were no trigger is generated so no mechanical excitation is conveyed into the tissue. Temporal correction is based on the main magnet drift subtraction using as baseline a gel filled vial within the FOV, which in our case was embedded into the 3D printed piston of our setup.
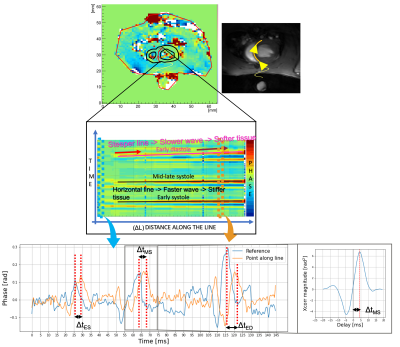
Figure 3. Schematics summarizing delay estimation via temporal signal cross-correlation on the waterfall diagrams data obtained from predefined lines that run through the septum myocardial tissue. Knowing time delay and spatial distance from the reference (closest point of the myocardium to the piston) one can easily estimate speed as 𝐶 ~ Δ𝐿/Δt.
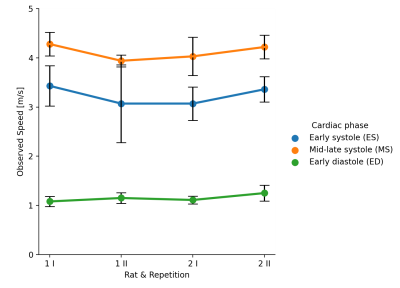
Figure 4. Preliminary biased speed results. Error bars show error on the mean.
DOI: https://doi.org/10.58530/2022/4789