4786
Multi-center evaluation of the novel Beat Sensor Cardiac triggering technology1Siemens Healthcare GmbH, Erlangen, Germany, 2Siemens Shenzhen Magnetic Resonance, Shenzhen, China, 3The Ohio State University, Columbus, OH, United States, 4Siemens Medical Solutions USA, Malvern, PA, United States, 5Jan Yperman Ziekenhuis, Ieper, Belgium, 6Royal Brompton Hospital, London, United Kingdom
Synopsis
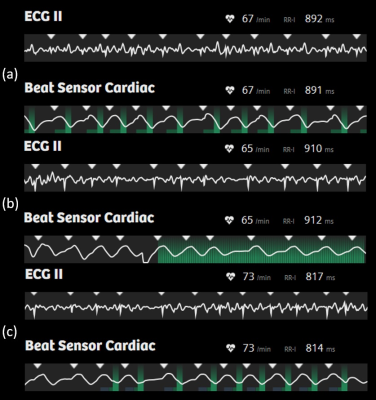
A multi-center evaluation of a Pilot Tone-based triggering technique using the BioMatrix Beat Sensor has been performed in healthy volunteers and patients undergoing a cardiac MRI examination. At five different sites, and two different field strengths, a wide range of MR sequences typically used in standard cardiac MRI examinations were successfully triggered using the prototype Beat Sensor Cardiac triggering technology. By including a short signal training and RF calibration step at the start of the examination, the device proved robust across a range of subjects, thereby offering an alternative means to trigger cardiac examinations without the need to attach electrodes.
Introduction
The Beat Sensor Cardiac triggering technology, which is the application of Pilot Tone to cardiac triggering, is a relatively new technique that allows for electrode-free triggering of cardiac MRI sequences (1 - 3). In this work we evaluated this technique at five MRI centers and at two field strengths in both patients and in healthy volunteers. We were interested in assessing the robustness of the method in different subject types, field strengths, and sequence flavors, as well as the stability of the technique with respect to workflow and usability.Methods
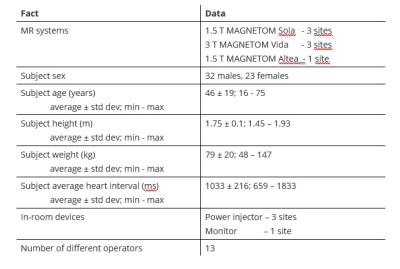
A prototype MR sequence and signal processing package was developed that allows for Beat Sensor Cardiac triggering of a wide range of MR sequence types typically used in standard cardiac MRI examinations. The pilot tone signal transmission and detection were performed using the commercially available BioMatrix Beat Sensor body array coil (Siemens Healthcare GmbH, Erlangen, Germany). The coil was positioned over the thorax, centered on the estimated heart position. The prototype software package 1) trains and calibrates the pilot tone signal, combining signals from all active local coil arrays and extracting the cardiac motion component, as well as correcting for signal corrupted e.g., during an RF pulse and 2) calculates a final filtered signal that is the inverted temporal derivative of the cardiac motion component. The trigger time point corresponds to the time of early systolic contraction and occurs ca. 200 ms after the R-wave. To facilitate the acquisition of static images of the heart in the end-diastolic phase of the cardiac cycle, the timepoint of the R-wave was estimated for each individual case from the cardiac motion component and used to adjust the timing parameters of the sequence protocol.The prototype was tested in a total of 55 subjects, of which 20 were patients, at five different sites and at two different field strengths (1.5 T, 3 T) running the software version syngo XA31A. Relevant for this evaluation were the subject sizes (height and weight) as well as the range of heart rates and heart rate variations, the presence of devices in the scanner room that could potentially interfere with the pilot tone signal and the number of different operators. Of the patients examined, indications included sarcoidosis, hypertrophic cardiomyopathy, myocarditis, chest pain, atrial fibrillation, among others. For reference purposes an ECG or pulse triggering device was attached and additional comparison data were acquired with ECG triggering in 30 subjects. Patients were positioned supine, head first in the scanner. All scanning facts are summarized in Figure 1.
Results
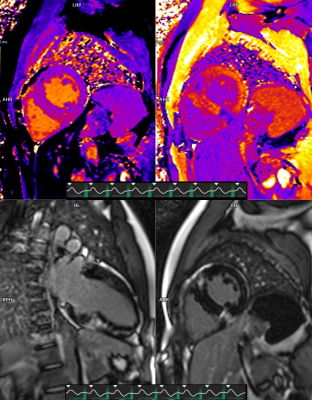
In all 55 subjects a wide range of standard clinical cardiac MR sequences were successfully triggered with the novel Beat Sensor Cardiac trigger technique. The exact type of sequences tested varied from examination to examination and site to site. However, in all cases at least localization - with single shot TrueFISP and dark-blood HASTE - and/or cine or late-enhancement sequences (TrueFISP) were performed and were accompanied by additional sequences depending on the clinical query or test protocol. In total, the following sequences were acquired successfully:- 47 cases cine
- 46 cases T1 mapping
- 27 cases T2 mapping
- 45 cases late-enhancement in free-breathing or breath-hold
- 21 cases dynamic sequences, of which 7 with contrast agent and one with adenosine
- 22 cases flow quantification in breath-hold or free-breathing
- 22 cases dark-blood prepared turbo spin echo both with and without STIR preparation.
Conclusions
We were able to demonstrate in a five-center evaluation of the Beat Sensor Cardiac triggering device that pilot tone-based triggering of standard cardiac examinations is feasible in a range of different patients and subject types and at different field strengths. This technology offers an alternative means to trigger clinical cardiac examinations without the need to attach electrodes, and, unlike ECG triggering, is robust to gradient interference.Acknowledgements
No acknowledgement found.References
1. Speier P, Fenchel M, Rehner R: Pt-nav: a novel respiratory navigation method for continuous acquisitions based on modulation of a pilot tone in the MR-receiver. Magn Reson Mater Phys Biol Med 28, 97{98 (2015)
2. Schroeder L, Wetzl J, Maier A, Lauer L, Bollenbeck J, Fenchel M, Speier P: A novel method for contact-free cardiac synchronization using the pilot tone navigator. In: Proceedings of the 24th Annual Meeting of ISMRM, Singapore, p. 410 (2016)
3. Bacher M, Speier P, Bollenbeck J, Fenchel M, Stuber M: Pilot tone navigation enables contactless prospective cardiac triggering: initial volunteer results for prospective cine. In: Intl. Soc. Mag. Reson. Med, vol.26, p. 4798 (2018)
4. Varghese J, Pan Y, Hayes C, Jin N, Simonetti 0, Speier P: Comparison of Beat Sensor Cardiac Triggering with ECG Triggering in a Comprehensive Cardiac MR Examination: Initial Volunteer Experience. submitted to SCMR 25th Annual Scientific Sessions (2022)
Figures