4784
B0 shimming for 7T cardiac T2*-weighted MRI in large animals: practical demands and hardware limitations.1Chair of Cellular and Molecular Imaging, Comprehensive Heart Failure Center, University Hospital Würzburg, Comprehensive Heart Failure Center, Wuerzburg, Germany, 2Department of Internal Medicine I, Cardiology, University Hospital Würzburg, Wuerzburg, Germany
Synopsis
Cardiac MRI with T2*-contrast at 7T is of particular interest because it is highly sensitive to cardiac tissue alterations after myocardial injury. A reliable T2*-contrast quantification and usage for tissue characterization require minimization of the inhomogeneity of the B0-field in the heart. In this work, we analyzed the B0-conditions achievable in longitudinal T2* measurements in the hearts of domestic pigs which during the experiment grew from 30 to 90 kg. B0 distribution statistics were summarized for the maps of individual slices. Demands on the 7T-scanners B0-shimming hardware (up to 3rd-order spherical harmonics) were analyzed.
Introduction
Cardiac MRI with T2*-contrast at 7T is of particular interest because of high sensitivity to cardiac tissue alterations after myocardial injury. This includes deposition of iron, hemorrhage, microvascular obstruction, and other factors influencing the local magnetic susceptibility [1,2]. The reliable quantification of a T2* for myocardial tissue characterization crucially requires minimizing the inhomogeneity of the B0-field in the heart, especially at the interfaces to the lung and liver tissue. The main goals of this study were: (1) to analyze the B0-conditions achievable in the longitudinal T2* measurements in the heart of the domestic pig of 30 to 90 kg weight range and (2) to find out the demands put by this B0-conditions for the 7T scanner B0-shimming hardware and limitations that are met.Methods
In total, the data of n=8 pigs (German Landrace) were used measured with the approval of the local Animal Welfare Committee (55.2 DMS 2532-1134-16, Government of Lower Franconia). All measurements were done using a Magnetom™ “Terra” 7T MR-scanner (Siemens, Erlangen, Germany) using custom-designed 8Tx/16Rx cardiac array coils adapted for a specific animal weight range. The scanner is equipped with a dedicated shimming system providing shim terms up to 3rd order spherical harmonics. The total shimming gradients configuration includes three linear (first-order) terms (X, Z, Y), five second-order terms (Z2, XY, ZX, ZY, X2-Y2), and four third-order terms (Z3, Z2X, Z2Y, Z(X2-Y2)).Each animal was scanned 4 times within 2 months while growing from 30-35 to 85-90kg. The acquisition of standard cardiac views: long-axis (LA), short-axis (SA), and two-chambers was done using the cardiac triggered (EasyACT, MRI.Tools system ) GRE-CINE sequence with the retrospective reconstruction of 30 cardiac phases. T2*-measurements and B0-mapping were performed using a triggered mGRE-sequence with 9 TE-times distributed in the range [1.1..14.6]ms. A contiguous stack of 10-13 short-axis view (SA) slices with 6mm thickness and in-plane pixel size of 2.2x2.5mm was acquired. By planning the CINE views, the B0-shimming volume was set to cover the whole visible heart volume. At the same time, for the T2* measurements, the shimming volume was adjusted on a slice-by-slice basis to force optimal B0 conditions. The reconstruction of B0 maps was done using ROMEO [3] software. Image segmentation and statistical analysis (including shimming protocol data) were done by in-house developed Matlab scripts (Mathworks, Natick, USA). The shim currents data were extracted retrospectively from the header of the DICOM files.
Results
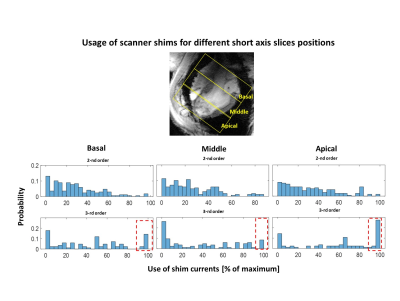
Figure 1 shows the examples of B0-maps in the whole SA-view of the heart and in the segmented left ventricle for the slices from basal to apical and covering different animal weights. Figure 2 shows the correlation between T2*-data (a) and B0-maps (b) for the slices at basal, mid-myocardium, and apical location. One can observe that increased localized B0-gradients influence significantly the T2* at corresponding segments of the myocardium. Figure 2c shows the statistics of residual inhomogeneity of B0 remaining after slice-by-slice shimming (computed over whole measured slices). Figure 3 compares the usage of shim currents for shimming volume placed over the whole heart and slice-by-slice basis. Figures 4 and 5 demonstrate the trends of the usage of shim coils currents for the slice-by-slice B0-shimming in pigs with different heart dimensions and averaged for the slices at different positions in the myocardium respectively.Discussion
The results presented in Figures 1 and 2 confirm the importance of the B0-shimming for the T2* quantification. Even after dedicated shimming performed for each slice the local gradients of high orders persist at the interfaces to the lung (mostly at the posterior and lateral heart walls). The strength of the local gradients increases in the apical direction with the local curvature of the myocardial wall. The localization of the high-order spherical harmonics gradients is in good agreement with earlier results of the study done in human subjects [4]. Figure 3 demonstrates that when the targeted shim volume is narrowed to the specific slice the demand on the compensation of the high order local gradients drastically increases. In about 50% of measured slices, the demanded current for 3rd order shimming meets the hardware limitations. The analysis of the shim currents demand for a pig of different weights shows that the most problematic challenge in terms of 3rd order shim currents is small hearts with a strong curvature. This demand decreases with the increasing size of the heart. Finally, the same trend is observed when considering demand on the 3rd order shimming in the slices at different positions ( Figure 5). The shim currents reach the hardware limit in up to 30% of the apical slices with the largest relative interface area to the lung tissue and strongest curvature.Conclusion
The T2* measurements at 7T covering the whole heart volume and especially apical regions represent a major challenge for the scanner’s B0-shimming system. The availability of the 3rd order shimming hardware is highly recommended for such studies. Slice-by-slice shimming in the majority of the cases meets the hardware limitations of the available 2nd and 3rd order shim amplifiers. Development of B0-shimming technologies based on the usage of the localized shimming coils [5] can open possibilities for further improvements of the B0-shimming quality in cardiac MRI at 7T.Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grants: 01EO1004, 01EO1504).References
[1] Ghugre, N.R., Ramanan, V., Pop, M., Yang, Y., Barry, J., Qiang, B., Connelly, K.A., Dick, A.J., Wright, G.A., 2011. Quantitative tracking of edema, hemorrhage, and microvascular obstruction in subacute myocardial infarction in a porcine model by MRI. Magnetic Resonance in Medicine 66, 1129–1141.. doi:10.1002/mrm.22855
[2] Elabyad, I.A., Terekhov, M., Lohr, D., Stefanescu, M.R., Baltes, S., Schreiber, L.M., 2020. A Novel Mono-surface Antisymmetric 8Tx/16Rx Coil Array for Parallel Transmit Cardiac MRI in Pigs at 7T. Scientific Reports 10.. doi:10.1038/s41598-020-59949-6
[3] Dymerska, B., Eckstein, K., Bachrata, B., Siow, B., Trattnig, S., Shmueli, K., Robinson, S.D., 2021. Phase unwrapping with a rapid opensource minimum spanning tree algorithm (ROMEO). Magnetic Resonance in Medicine 85, 2294–2308.. doi:10.1002/mrm.28563
[4] Hock, M., Terekhov, M., Stefanescu, M.R., Lohr, D., Herz, S., Reiter, T., Ankenbrand, M., Kosmala, A., Gassenmaier, T., Juchem, C., Schreiber, L.M., 2021. B 0 shimming of the human heart at 7T. Magnetic Resonance in Medicine 85, 182–196.. doi:10.1002/mrm.28423
[5] Juchem, C., Theilenberg, S., Kumaragamage, C., Mullen, M., Delabarre, L., Adriany, G., Brown, P.B., Mcintyre, S., Nixon, T.W., Garwood, M., Graaf, R.A., 2020. Dynamic multicoil technique (DYNAMITE) MRI on human brain. Magnetic Resonance in Medicine 84, 2953–2963.. doi:10.1002/mrm.28323
Figures