4777
Development of a Prospectively Motion Corrected Free-breathing FLASH Sequence1Department of Human Biology, Division of Biomedical Engineering, University of Cape Town, Cape Town, South Africa, 2Cape Universities Body Imaging Center, University of Cape Town, Cape Town, South Africa
Synopsis
Respiratory motion of the heart is a fundamental challenge to cardiac MR imaging (CMR), frequently compensated for with breath-holding and acceptance-window methods. These methods are not always viable and result in inefficient acquisitions, creating longer scan times1. Here, an adaptive Kalman-filter with a control system was implemented in a FLASH sequence to update the position of the slice during the imaging segment based on repeated navigator measurements acquired during the non-imaging segment. This predictive tracking results in a free-breathing sequence which is less strenuous for subjects, more efficient and reduces scan times.
Introduction
Respiration causes severe image degradation in CMR. A common correction method uses diaphragmatic navigators to accept only information acquired when the diaphragm is within certain window positions2. This substantially increases the acquisition time. Here we implement a prospective motion correction control system based on an adaptive Kalman filter in a FLASH sequence3. Based on diaphragm positions measured during non-imaging segments, the Kalman filter predicts the position of the diaphragm during the imaging segment. The position of the slice is then prospectively adjusted using a linear scaling factor. Typically, a generalized scaling factor of 0.6 is used but this does not compensate for the variation amongst subjects nor the 3D nature of the heart.4Methods
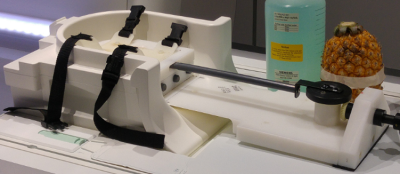
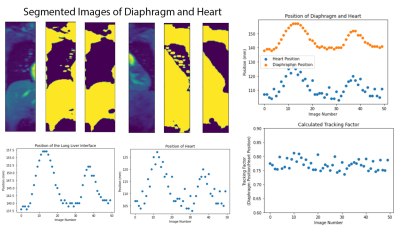
The FLASH sequence was modified to include multiple navigator readings between imaging segments. Initially a set of 256 diaphragm positions is acquired to pre-train the Kalman filter. Before each imaging segment navigator positions are gathered and used as the input into the control system. The predicted outputs of the control system are then used for slice following during the imaging segment, during which no navigator information is available. Imaging was tested with phantoms initially and followed by a set of in vivo scans from 8 healthy volunteers. All imaging was performed on a 3T Skyra (Siemens AG, Erlangen). Initial phantom testing was performed utilizing a motion rig (Figure 1) that simulates breathing motion. Five sets of ECG-triggered FLASH acquisitions were performed in each healthy volunteer: (i) breath holds (BH)s, (ii) free-breathing with no motion correction, (iii) free-breathing-navigated-FLASH with a 4mm acceptance window, (iv) free-breathing FLASH adapted to utilize the control system, and (v) a set of low-resolution cine FLASH images (TR=86ms, 50 images). The log data from the acquisitions with the control system adapted sequence were then analysed to measure the accuracy of the control system’s predictions. Images acquired with the standard BH sequence were compared to the control system adapted sequence, the acceptance window slice following sequence and the uncorrected free-breathing sequence. Finally, the set of cine images were segmented at the lung-liver interface and around the heart. The edge of the lung liver interface and an edge of the heart were tracked to calculate the proportional change of the diaphragm’s position to the heart’s position, for each subject.Results
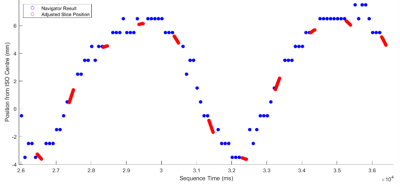
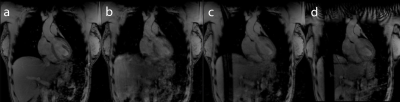
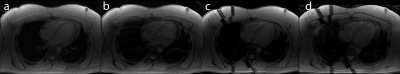
Figure 2 shows the control system’s predictions for the diaphragm position during the imaging segments (red), given the diaphragm positions measured by the navigator during non-imaging segments (blue). Figures 3 and 4 show example images from uncorrected breathing, free-breathing windowed, free-breathing control system corrected acquisitions. Tracking the edge of the lung-liver interface and the heart yielded variable tracking factors across subjects (0.65 to 0.77). For the subject shown, the tracking scaling factor was 0.77.Discussion
Although the position tracking of the control system is accurate, tracking during non-linear sections of the breathing cycle remains challenging. The resultant images show improved quality using the control system compared with no correction and similar quality when compared with the windowed acquisition, although artefacts due to the expansion and contraction of the chest wall remain.Conclusion
The control system adapted acquisition provides similar image quality to the windows acquisition, whilst maintaining 100% imaging efficiency through the respiratory cycle. There is clear evidence for variation in the tracking scaling factor amongst subjects and therefore a need for subject specific adaptation when considering navigated sequences.Acknowledgements
No acknowledgement found.References
1. Holland, A. E., Goldfarb, J. W., & Edelman, R. R. (1998). Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology, 209(2), 483–489.
2. Sachs, T. S., Meyer, C. H., Hu, B. S., et al. (1994). Real-time motion detection in spiral MRI using navigators. Magnetic Resonance in Medicine, 32(5), 639–45.
3. Taylor, A. M., Keegan, J., Jhooti, P., Firmin, D. N., & Pennell, D. J. (1999). Calculation of a subject-specific adaptive motion-correction factor for improved real-time navigator echo-gated magnetic resonance coronary angiography. J Cardiovasc Magn Reson, 1 (2), 131{138. doi: 10.3109/10976649909080841
4. Burger, I. H., Keegan, J., Meintjes, E., et al. Prospective diaphragm position prediction for Cardiac MR using multiple navigators. Proc ISMRM-ESMRMB 18, ISSN# 1545-4428, Stockholm, Sweden, 1-7 May 2010. (#5013)
Figures