4773
Relationship between Microvascular Obstruction and Regional Myocardial Strain by CMR after ST-segment-elevation Myocardial Infarction
Yanan Zhao1, Jianing Cui1, Xueqian Liu2, Tao Li1, and Xiuzheng Yue3
1Department of Radiology, Chinese People’s Liberation Army General Hospital, Beijing, China, 2Qinhuangdao Workers' Hospital, Qinhuangdao, China, 3Philips Healthcare, Beijing, China
1Department of Radiology, Chinese People’s Liberation Army General Hospital, Beijing, China, 2Qinhuangdao Workers' Hospital, Qinhuangdao, China, 3Philips Healthcare, Beijing, China
Synopsis
Effective risk assessment and stratification are essential for the clinical management of ST-segment elevation myocardial infarction (STEMI) patients. CMR imaging has become a beneficial imaging modality to assess myocardial morphology, function and infarct characteristics simultaneously. This study quantitatively evaluated relationship between microvascular obstruction and regional myocardial strain by Cardiac MRI after ST-segment-elevation myocardial infarction. Our data showed that regional strain parameters may be s new noninvasive imaging markers allowing comprehensive evaluation regional myocardium deformation.
Introduction
Effective risk assessment and stratification are essential for the clinical management of ST-segment elevation myocardial infarction (STEMI) patients1-3. Recently, CMR feature tracking (FT), a novel postprocessing method, allows to measure myocardial strain by tracking tissue voxel motion in steady state free procession cine images without additional sequences to acquire images and demonstrate greater robustness and reproducibility.4,5 The aim of study is to investigate in detail MVO impact on regional function in radial, circumferential and longitudinal directions in a large group of patients with revascularized STEMI by CMR-FT technique.Materials and Methods
CMR images were retrospectively studied in 157 STEMI patients with who underwent CMR at 1-7 days after successfully reperfusion (mean age 61.44 ± 11.5 years) were included in this prospective study. CMR scans was performed on 1.5T MR scanner (Multiva, Philips, Netherlands) with a 18-element-body phased-array coil system. Cardiac function was evaluated by balanced turbo field echo (BTFE) cine sequence at the continuous short-axis covering the whole left ventricle (LV) and the long-axis (2/3/4 chamber) views under breath-holding. Imaging parameters included TR/TE 3.7/1.8ms, in-plane resolution 1.4 × 1.4mm2, flip angle 60° and slice thickness 8mm with 8 to 12 slices gathered on the short-axis. Late gadolinium enhancement (LGE) imaging was performed 10 to 15 minutes after administration of 0.2mmol/kg gadolinium-based contrast agent (Gadopentetate Dimeglumine, BeiLu, Beijing, China) using a segmented phase-sensitive inversion-recovery fast gradient-echo pulse sequence (PSIR). The slice location was consistent with the cine sequence. Other imaging parameters included TR/TE 6.2/3ms, in-plane resolution 1.6×1.65mm2, flip angle 25° and slice thickness 8mm. The parameters of LV volumes and function were calculated using CVI42 (Circle Cardiovascular Imaging, Calgary, Canada). The infarct, adjacent and remote regional strain parameters in radial, circumferential and longitudinal directions were measured in 16-segment model by CMR-FT from cine images. Infarction and MVO zones were defined on Late gadolinium enhancement (LGE) images (Figure 1). The regional strain in patients with and without MVO was compared using independent-samples t-test. Diagnostic performance was assessed by analyzing the area under the receiver operating characteristic (AUC). A patient with MVO have shown in Figure 2. Shapiro-Wilk test was used to assess the normality of continuous variables. Continuous variables between groups were compared using t-test, one-way ANOVA, Kruskal-Wallis H test, as appropriate. Categorical variables were compared using chi-square test. Spearman or Pearson correlation coefficients were calculated between continuous variables, as appropriate. P<0.05 was considered statistically significant.Results
There are 157 STEMI patients were recruited. MVO was present in 36% (56 of 157) of patients. Patients with MVO had reduced peak strain and corresponding systolic and diastolic strain rate in radial (P < .001, P = .004, P < .001) and circumferential (P <.001, P = .04 and P = .01) directions in infarct segments than those without MVO (Figure 3). In the infarct segments with MVO, peak strain (all P < .001) and peak diastolic strain rate (p = .003, p < .001 and P = .003) (Figure 3)in three directions and peak systolic strain rate (all P < .001) (Figure 4) in radial and circumferential directions were impaired compared with those without MVO, whereas the corresponding AUCs of strain were less than 0.7. (Figure 5)Conclusions
Regional strain parameters derived with CMR-FT may be new noninvasive imaging markers with allowed comprehensive evaluation regional myocardium deformation. MVO deteriorates the function of the infarct segments in STEMI patients. However, these strain paraments had poor diagnostic performance in differentiating infarct segments with and without MVO.Acknowledgements
No acknowledgement found.References
1 Freisinger E, Fuerstenberg T, Malyar NM, et al. German nationwide data on current trends and management of acute myocardial infarction: discrepancies between trials and real-life. Eur Heart J 2014;35:979–88. 2 Roffi M, Patrono C, Collet JP, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. 3 O’Gara PT , Kushner FG, Ascheim DD, et al. CF/AHA T ask Force. 2013 ACCF/AHA guideline for the management of ST -elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association T ask Force on Practice Guidelines. Circulation 2013;127:529–555. 4. Wamil M, Borlotti A, Liu D, et al. Combined T1-mapping and tissue tracking analysis predicts severity of ischemic injury following acute STEMI-an Oxford Acute Myocardial Infarction (OxAMI) study. Int J Cardiovasc Imaging. 2019;35(7):1297-308. 5. Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol. 2015;84(5):840-48.Figures
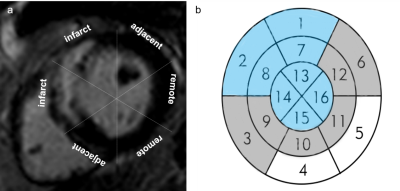
Figure 1 The division of the infarct, adjacent and
remote segments. Definition of the infarct, adjacent and remote zone myocardium
in a representative short axis image of a patient with anterior myocardial
infarction by LGE (a). Schematic diagram of the infarct, adjacent and remote
zone (b). If the infarct zone included the segments 1, 2, 7, 8, 13, 14, 15
and16, the adjacent zone were the segments 3, 6, 9,10, 11 and 12, and the rest
was defined as the remote zone
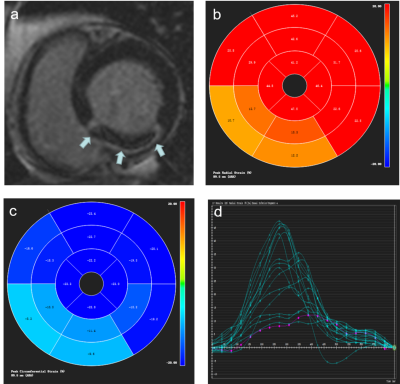
Figure 2 Images in a
54-year-old man with microvascular obstruction (MVO). Typical example of Short axis image of inferior
myocardial infarction with microvascular obstruction (MVO) by LGE imaging (a).
Representative bull’s eye maps for radial and circumferential strain
assessment, respectively (b and c). The cvi42-derived tissue tracking analysis
about radial peak strain of 16 segments (d).
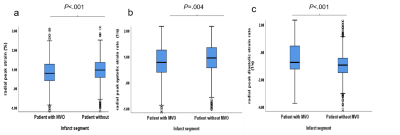
Figure 3 The box diagram of the LV myocardium radial
peak strain (a), radial peak systolic strain rate (b) and radial peak diastolic
strain rate (c) within infract segments in patient with or without MVO
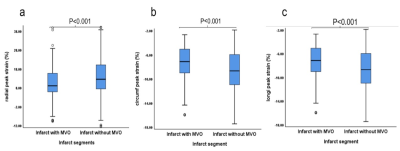
Figure 4 The box diagram of the LV myocardium radial
peak strain (a), circumferential peak strain (b) and longitudinal peak strain (c)
within infarct segments with or without MVO.
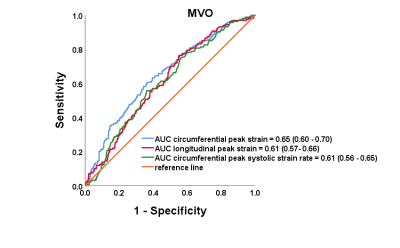
Figure 5
Receiver-operator characteristic curves of
circumferential peak strain, longitudinal peak strain and peak circumferential
systolic strain rate.
DOI: https://doi.org/10.58530/2022/4773