4769
Brain Mapping of Mindfulness Meditation, Slow Paced Breathing and Spontaneous Breathing1Martinos Center, Radiology, Massachusetts General Hospital, Charlestown, MA, United States
Synopsis
A preliminary comparison of physiological and fMRI data from an experienced meditator during mindfulness, paced breathing at 6 breaths/min, and spontaneous breathing provided several characteristic findings related to the brain-body mechanisms of mindfulness: 1) a reduction of breathing rate together with indicators of improved respiratory gas exchange (RGE); 2) a significant correlation between the temporal oscillation of RGE metrics and cerebral hemodynamic fluctuations (CHF) in regions within the salience and default mode networks; 3) a difference in the coherence of CHF with RGE and with heart cycle duration (NN).
Introduction
Our objective is to study the physiological mechanism of brain-body interaction between respiratory gas exchange (RGE) and brain activities during mindfulness (short for mindfulness meditation) in experienced meditation practitioners. Mindfulness involves attention to breathing. It reduces breathing rate naturally to around 6 breaths/min1, 2. Paced breathing at 6 breaths/min has been reported to improve RGE3-5, and motivates the investigation of the role of RGE in mindfulness. RGE metrics include oxygen (O2) uptake and carbon dioxide (CO2) release and breath-by-breath O2-CO2 exchange ratio (bER)6, which takes into account the effect of both O2 uptake and CO2 release. These metrics were reported to be superior to respiratory rate/volume in correlating with brain activities6. The main question is: Is the brain-body mechanism of mindfulness attributable primarily to the mechanism of slow-paced breathing of around 6 breaths/min? As inspiration and expiration strongly impact the change of heart rate7, we also explored how cerebral hemodynamic fluctuations (CHF) correlated with heart cycle duration (NN).Subjects and Methods
An experienced meditation practitioner participated in an fMRI scan during three conditions: 1) mindfulness; 2) paced breathing at 6 breaths per minute; 3) spontaneous breathing. MRI was performed on a 3-Tesla (Siemens Medical, Erlangen, Germany). Whole-brain BOLD-fMRI datasets were acquired for 10 minutes (TR=1250ms, TE=30ms). Physiological data including respiration, EKG, partial pressure of O2 (PO2), and CO2 (PCO2) were collected simultaneously with MRI acquisition. Data analysis: BOLD-fMRI data were imported into the software AFNI8. The physiological data were analyzed using Matlab R2020a. The RGE metrics of DPO2, DPCO2, and bER were calculated using the same procedures as described in our previous studies6. Cardiovascular metrics included normal heart cycle duration (NN), NN variability related to heart rate variability (HRV). For each of the 3 conditions (mindfulness, paced breathing, and spontaneous breathing), BOLD signal changes (DBOLD) were cross-correlated separately with bER and NN using Hilbert Transform analysis. A significant correlation was considered at corrected p<0.05. Wavelet transform coherence analysis was used to measure the dynamic correlation of global DBOLD with RGE metrics and NN in the time-frequency domain6.Results
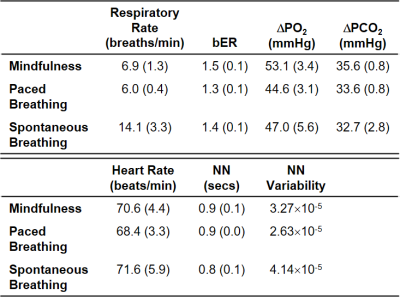
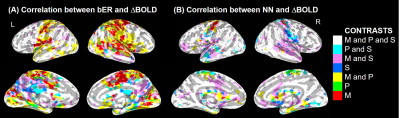
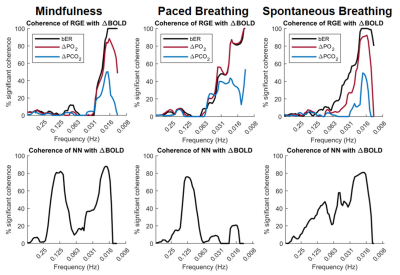
Mindfulness lowered the breathing rate naturally to close to 6 breaths per minute (Table 1). bER had the highest value during mindfulness, where there was a simultaneous rise of O2 uptake (DPO2). NN was similar among the three conditions, while the NN variability decreased from spontaneous breathing to mindfulness and then to slow-paced breathing. Multiple brain regions common in mindfulness and slow-paced breathing (Figure 1A; M and P, in yellow) showed a significant bER-DBOLD correlation. Regions with significant bER-DBOLD correlation (Figure 1A; M, in red) was also found only during mindfulness. Mindfulness shared the significant bER-DBOLD correlation with spontaneous breathing but not with slow-paced breathing at the posterior cingulate and precuneus (Figure 1A; M and S, in purple). NN-DBOLD correlation maps were also reported (Figure 1B), indicating that bER and NN interact differently with DBOLD in the three conditions. RGE metrics were shown to oscillate with DBOLD at a very low frequency of 0.008-0.063Hz in all three conditions (Figure 2), while NN oscillated with DBOLD at both a low frequency of 0.063-0.25Hz and a very low frequency of 0.008-0.063Hz during mindfulness and spontaneous breathing. NN oscillated with DBOLD mainly at the frequency band of 0.063-0.25Hz during slow-paced breathing (Figure 2).Discussion
Although the salience and default mode networks have been reported during mindfulness9, 10, to our knowledge, no previous study reported their physiological relationship with RGE. Common brain regions showing significant bER-DBOLD correlation during mindfulness and slow-paced breathing (Figure 1A) explain perhaps the shared features of slow breathing and mindfulness. Posterior cingulate and precuneus regions common for mindfulness and spontaneous breathing but not for slow-paced breathing in their significant bER-DBOLD correlation (Figure 1A) suggest that the internal attention to breathing during mindfulness is different from the attention to external pacing stimuli. The findings in Figure 2 suggest that the bER-DBOLD and NN-DBOLD correlations have different frequency distributions. It is also not surprising that NN variability (Table 1) is smallest for slow-paced breathing but largest for spontaneous breathing. Steffen et al.11 reported reduced HRV at high (respiratory) frequencies but increased HRV at low frequencies for 6 breaths/min. It suggests that potential health benefits can involve the modulation of both the parasympathetic and sympathetic systems. A reduction of HRV by attention12 may partly explain why mindfulness also has smaller NN variability than spontaneous breathing.Conclusion
Our data show that mindfulness differs from slow-paced breathing and from spontaneous breathing in their brain-body interaction, which can be studied with fMRI to map the correlation of brain activities with RGE and cardiovascular metrics.Acknowledgements
This research was carried out at the Athinoula A. Martinos Center at the Massachusetts General Hospital, using resources provided by NCCIH grant R21AT010955 and by NIH grant P41EB015896 of Center for Functional Neuroimaging Technologies.References
1. Bernardi L, Sleight P, Bandinelli G, et al. Effect of rosary prayer and yoga mantras on autonomic cardiovascular rhythms: comparative study. BMJ 2001;323:1446-1449.
2. Wielgosz J, Schuyler BS, Lutz A, et al. Long-term mindfulness training is associated with reliable differences in resting respiration rate. Sci Rep 2016;6:27533.
3. Bernardi L, Spadacini G, Bellwon J, et al. Effect of breathing rate on oxygen saturation and exercise performance in chronic heart failure. Lancet 1998;351:1308-1311.
4. Bilo G, Revera M, Bussotti M, et al. Effects of slow deep breathing at high altitude on oxygen saturation, pulmonary and systemic hemodynamics. PLoS One 2012;7:e49074.
5. Sin PY, Webber MR, Galletly DC, et al. Interactions between heart rate variability and pulmonary gas exchange efficiency in humans. Exp Physiol 2010;95:788-797.
6. Chan ST, Evans KC, Song TY, et al. Dynamic brain-body coupling of breath-by-breath O2-CO2 exchange ratio with resting state cerebral hemodynamic fluctuations. PLoS One 2020;15:e0238946. 7. Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 2007;74:263-285.
8. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996;29:162-173.
9. Ramirez-Barrantes R, Arancibia M, Stojanova J, et al. Default Mode Network, Meditation, and Age-Associated Brain Changes: What Can We Learn from the Impact of Mental Training on Well-Being as a Psychotherapeutic Approach? Neural Plast 2019;2019:7067592.
10. Holzel BK, Lazar SW, Gard T, et al. How Does Mindfulness Meditation Work? Proposing Mechanisms of Action From a Conceptual and Neural Perspective. Perspect Psychol Sci 2011;6:537-559.
11. Steffen PR, Austin T, DeBarros A, et al. The Impact of Resonance Frequency Breathing on Measures of Heart Rate Variability, Blood Pressure, and Mood. Front Public Health 2017;5:222.
12. Gazzellini S, Dettori M, Amadori F, et al. Association between Attention and Heart Rate Fluctuations in Pathological Worriers. Front Hum Neurosci 2016;10:648.
Figures