4763
Functional connectome of hypothalamus, nucleus accumbens and hippocampus in living humans from 7 Tesla resting-state fMRI1Institute of Life Sciences, Scuola Superiore Sant'Anna, Pisa, Italy, 2Research Center E. Piaggio, University of Pisa, Pisa, Italy, 3Department of Radiology, Athinoula A. Martinos Center for Biomedical Imaging (MGH), Charlestown, MA, United States, 4Escuela Nacional de Estudios Superiores, Juriquilla, Universidad Nacional Autónoma de México, Queretaro, Mexico, 5Division of Sleep Medicine, Harvard University, Boston, MA, United States
Synopsis
The hypothalamus and nucleus accumbens promote respectively wakeful arousal and sleep, producing a stable cycle between states. The hippocampus is inherently connected to this cycle, which modulates memory encoding during wakefulness and sleep. While their connectivity to cortical regions is detailed in literature, their connectivity with brainstem nuclei is understudied in living humans.
By using high spatial resolution 7 Tesla resting state fMRI and an in-vivo brainstem nuclei atlas, we provided a functional connectome of hypothalamus, nucleus accumbens and hippocampus with the rest of the brain, including 58 brainstem nuclei along with 148 cortical and 25 subcortical structures.
Introduction
Subcortical nuclei play a key role in the integration of fundamental functions with higher-order cortical processing. Studying their connectivity is crucial to understand how different networks in the brain cooperate to maintain energy homeostasis, store information, and stabilize sleep/wake cycles. The hypothalamus and the nucleus accumbens have long been recognized as competitors promoting respectively wakefulness and slow-wave sleep1,2, and the latter is also crucially involved in reward/motivation. Hippocampus activity is strongly modulated by these state changes: it correlates with the well-known REM-sleep waves3 and it plays a prominent role in memory encoding4. Other studies have investigated the connectivity of these nuclei, predominantly with cortical and subcortical regions5–7; yet, their connectivity with brainstem nuclei is understudied due to difficulty in localizing these nuclei in conventional MRI.Purpose
To apply our recently developed in-vivo brainstem nuclei atlas8–12 to 7 Tesla high spatial resolution resting-state fMRI, and map the functional connectivity of hypothalamus, nucleus accumbens and hippocampus with 58 brainstem nuclei along with 148 cortical and 35 subcortical structures.Methods
Twenty healthy volunteers (10m/10f; age 29.5±1.1years, underwent 7 Tesla and 3 Tesla MRI under IRB approval.7 Tesla MRI: fMRI data acquisition: three resting-state (eyes-closed) fMRI runs were acquired with parameters: gradient-echo EPI, isotropic voxel-size/matrix-size/GRAPPA factor/nominal echospacing/bandwidth/N. slices/slice orientation/slice-acquisition order/echo-time (TE)/repetition-time (TR)/flip-angle(FA)/simultaneous-multi-slice factor/N. repetitions/phase-encoding direction/acquisition-time per-run=1.1mm/180x240/3/0.82ms/“1488Hz/Px”/123/sagittal/interleaved/32ms/2.5s/75°/3/210/anterior-posterior/10’07”. Fieldmap: isotropic voxel-size/matrix-size/bandwidth/N. slices/slice orientation/slice-acquisition order/TE1/TE2/TR/FA/simultaneous-multi-slice factor/phase-encoding direction=2.0mm/116x132/”1515Hz/Px”/80/sagittal/interleaved/3.00ms/4.02ms/570.0ms/36°/3/anterior-posterior.
3 Tesla MRI: T1-weighted MEMPRAGE data acquisition: isotropic voxel-size/TR/TEs/inversion-time/FA/FOV/bandwidth/GRAPPA-factor/slice orientation/slice-acquisition order/acquisition time=1mm/2.53s/1.69,3.5,5.3,7.2ms/1.5s/7°/256x256x176mm3/“650Hz/pixel”/3/sagittal/anterior-posterior/4′28”.
Preprocessing
MEMPRAGEs: We computed the root-mean-square MEMPRAGE across echo-times, rotated it to standard “RPI” orientation, performed bias-field correction (SPM) and cropping. Then, we computed (ANTs) a group-average MEMPRAGE template as an intermediate step to coregister single-subject MEMPRAGEs to Montreal-Neurological-Institute (MNI) space. The pre-processed T1-weighted images were parcellated using Freesurfer to generate cortical and subcortical targets.
fMRI: To remove physiological noise we applied RETROICOR to fMRI data. Images were slice-timing corrected, reoriented to “RPI” orientation, and cropped. Then, we computed the transformations to correct for geometric distortions (using the fieldmap) and for rigid-body motion. The bias-field-corrected time-averaged fMRI of the first run was used to compute an affine transformation and a nonlinear warp for coregistration to the MEMPRAGE. After concatenating and applying transformations relative to distortion-, motion-correction and coregistration to the MEMPRAGE, we regressed out nuisance time-series due to motion, cardiac rate and respiratory-volume-per-unit-time fluctuations, and signal in the cerebro-spinal fluid neighboring the brainstem. We scaled the signal to percent signal change, removed the temporal mean, and performed band-pass filtering (cut-off 0.01-0.1 Hz). Finally, we concatenated the runs and applied the MEMPRAGE-to-MNI transformations.
Seed and target regions: We used as seeds an atlas of hypothalamus13 and the Freesurfer segmentation of bilateral nucleus accumbens and hippocampus14. As targets we used the whole set of 231 cortical14, subcortical14 and brainstem nuclei8–12 masks.
Region-based connectivity analysis: At the subject level, the Pearson’s correlation coefficient was computed between average time-courses extracted from seeds and targets. At the group level, we performed a one-sample t-test on the Fisher-transformed correlation coefficients and defined as ‘links’ significant connections.
Voxel-based connectivity analysis: We used AFNI functions (3dDeconvolve, 3dTtest++) to perform regression analysis for each seed timeseries at the subject and group level. T-statistics were transformed to -log10(p-values), and a cluster-size based threshold to control for false-positives was defined from the autocorrelation-function of residuals (AFNI 3dClustSim). Results were displayed on volumetric slices and also projected to medial and lateral Freesurfer surfaces.
Results
In Figures 1-3, for hypothalamus, nucleus accumbens and hippocampus we display in A) their region-based functional connectome with the rest of the brain (p<0.0005, Bonferroni corrected); in B) their voxel-based functional connectivity maps projected with FreeSurfer on the medial and lateral cortex of both hemispheres; in C) the voxel-based functional connectivity maps on three orthogonal views (with FSLview), centered on the center of mass of the seed (seed contour displayed in green). For the voxel-based analysis, for display purposes, we used the following thresholds: for nucleus accumbens and hypothalamus per-voxel p=0.0001, cluster-wise p=0.05; for hippocampus per-voxel p=0.00001, cluster-wise p=0.01.Discussion
In line with animal literature1–3, our results indicated high interconnectivity of hypothalamus and nucleus accumbens within the arousal network and sleep-regulating centers, including brainstem arousal/sleep nuclei; nucleus accumbens also displayed connectivity with nuclei involved in reward/motivation. The hippocampus was functionally connected with regions involved in memory encoding (amygdala, prefrontal cortex), as well as, interestingly, with brainstem nuclei involved in arousal/motor/sensory function, which might modulate memory processes. Limitations of this study include the possible presence of residual physiological noise, and the polysynaptic nature of the Pearson correlation coefficient, to be tackled in further investigations.Conclusion
We provided functional connectomes of hypothalamus, nucleus accumbens and hippocampus, confirming several connections at the basis of sleep-state regulation and memory fixation. These connectomes might provide a baseline to investigate sleep and memory disorders.Acknowledgements
NIH NIA-R01AG063982References
1. Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24(12):726-731. doi:10.1016/S0166-2236(00)02002-6
2. Valencia Garcia S, Fort P. Nucleus Accumbens, a new sleep-regulating area through the integration of motivational stimuli. Acta Pharmacol Sin. 2018;39(2):165-166. doi:10.1038/aps.2017.168
3. Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep State Switching. Neuron. 2010;68(6):1023-1042. doi:10.1016/j.neuron.2010.11.032
4. Spoormaker VI, Czisch M, Holsboer F. REM sleep, hippocampus, and memory processing: insights from functional neuroimaging studies. Behav Brain Sci. 2013;36(6):629-630; discussion 634-59. doi:10.1017/S0140525X13001441
5. Kullmann S, Heni M, Linder K, et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp. 2014;35(12):6088-6096. doi:10.1002/hbm.22607
6. Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional Connectivity and Coactivation of the Nucleus Accumbens: A Combined Functional Connectivity and Structure-Based Meta-analysis. J Cogn Neurosci. 2011;23(10):2864-2877. doi:10.1162/jocn.2011.21624
7. Ezama L, Hernández‐Cabrera JA, Seoane S, Pereda E, Janssen N. Functional connectivity of the hippocampus and its subfields in resting‐state networks. Eur J Neurosci. 2021;53(10):3378-3393. doi:10.1111/ejn.15213
8. Bianciardi M, Toschi N, Edlow BL, et al. Toward an In Vivo Neuroimaging Template of Human Brainstem Nuclei of the Ascending Arousal, Autonomic, and Motor Systems. Brain Connect. 2015;5(10):597-607. doi:10.1089/brain.2015.0347
9. Bianciardi M, Strong C, Toschi N, et al. A probabilistic template of human mesopontine tegmental nuclei from in vivo 7T MRI. Neuroimage. 2018;170:222-230. doi:10.1016/j.neuroimage.2017.04.070
10. García-Gomar MG, Strong C, Toschi N, et al. In vivo Probabilistic Structural Atlas of the Inferior and Superior Colliculi, Medial and Lateral Geniculate Nuclei and Superior Olivary Complex in Humans Based on 7 Tesla MRI. Front Neurosci. 2019;13. doi:10.3389/fnins.2019.00764
11. Singh K, Indovina I, Augustinack JC, et al. Probabilistic Template of the Lateral Parabrachial Nucleus, Medial Parabrachial Nucleus, Vestibular Nuclei Complex, and Medullary Viscero-Sensory-Motor Nuclei Complex in Living Humans From 7 Tesla MRI. Front Neurosci. 2020;13. doi:10.3389/fnins.2019.01425
12. Singh K, García-Gomar MG, Bianciardi M. Probabilistic Atlas of the Mesencephalic Reticular Formation, Isthmic Reticular Formation, Microcellular Tegmental Nucleus, Ventral Tegmental Area Nucleus Complex, and Caudal–Rostral Linear Raphe Nucleus Complex in Living Humans from 7 Tesla Magnetic Reso. Brain Connect. 2021;11(8):613-623. doi:10.1089/brain.2020.0975
13. Pauli WM, Nili AN, Tyszka JM. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci Data. 2018;5(1):180063. doi:10.1038/sdata.2018.63
14. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1-15. doi:10.1016/j.neuroimage.2010.06.010
Figures
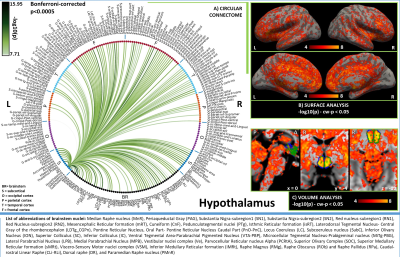
Figure 1. A) Region-based functional connectome and B)-C) voxel-based functional connectivity maps of hypothalamus. We observed bilateral connectivity of hypothalamus with regions involved in: (i) arousal, i.e. VTA-PBP, LDTg-CGPn, LC (weaker unilateral), LPB (unilateral), MPB, serotonergic MnR, PMnR, DR, CLi-RLi along with bilateral thalamus, accumbens and frontal cortex; (ii) arousal/motor function. i.e. left SN1, SN2, SubC, PTg, and bilateral PnO-PnC.
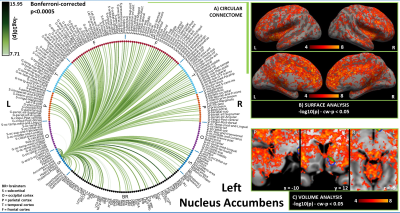
Figure 2. A) Region-based functional connectome and B)-C) voxel-based functional connectivity maps of nucleus accumbens (list of abbreviations as in Figure 1). We observed connections with LDTg-CGPn, DR, PMnR, PTg, PAG, SubC, PnO-PnC, isRt, mRt and with thalamus, hypothalamus, insular and frontal cortex, involved in arousal and/or REM sleep; with VTA, and PAG in line with its role in motivation/reward; and with pallidum in line with inhibitory efferent links to wake-promoting areas2.
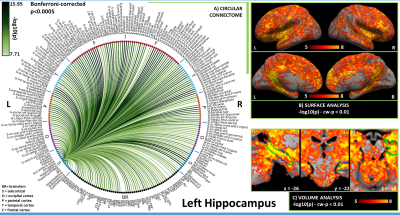
Figure 3. A) Region-based functional connectome and B)-C) voxel based functional connectivity maps of hippocampus (list of abbreviations as in Figure 1). The strongest connections were observed in the brainstem with arousal/motor nuclei (SN2, RN2, LDTg-CGPn, iMRt —also bilateral yet weaker connectivity with LC) and sensory nuclei (IC, MITg-PBG, Ve, VSM); in the subcortex with LG, MG, amygdala and putamen; in the cortex with regions belonging to the default mode network, in line with literature7.