4759
Intra-hippocampal fiber tracking and connectome from submillimeter DTI of the human hippocampus in vivo and ex vivo1Brain Imaging and Analysis Center, Duke University, Durham, NC, United States, 2Medical Physics Graduate Program, Duke University, Durham, NC, United States, 3Department of Radiology, Duke University, Durham, NC, United States, 4Department of Neurology, Duke University, Durham, NC, United States, 5Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital, Taoyuan, Taiwan
Synopsis
The hippocampus plays an essential role in memory; the impairment in intra-hippocampal connectivity could result in memory loss in neurodegenerative dementias. We generate intra-hippocampal fiber tracts and connectomes from submillimeter isotropic DTI images for repeated scans from the same subjects and across different subjects. We characterize the fiber orientations and connectivity across hippocampal subfields by registering all in vivo scans onto the population template and we compare these results with those from an ex vivo human brain sample. This method can assess intra-hippocampal connectivity in vivo, which could be potentially used to diagnose neurodegenerative diseases.
Introduction
The hippocampus plays an important role in memory. Changes in neuronal connectivity within the hippocampus could result in memory impairment in neurodegenerative dementias such as Alzheimer’s disease (AD)1. Previous DTI studies have used fiber tracking to investigate the connectivity from the hippocampus to other brain regions in vivo2 and intra-hippocampal connectivity in ex vivo human3,4 and animal5 brain samples. However, it has been challenging to achieve a submillimeter spatial resolution within a reasonable scan time to study intra-hippocampal connectivity in the human hippocampus in vivo. Here, we use a multi-band multiplexed sensitivity-encoding (MB-MUSE) technique6,7 to acquire submillimeter whole-brain DTI data in vivo under 24 minutes. We generate intra-hippocampal fiber tracts and connectomes for repeated scans from the same subjects and for different subjects, characterize the fiber orientations and connectivity across hippocampal subfields, and compare these results with those from an ex vivo human brain sample, with the long-term goal of providing a tool to assess the integrity of intra-hippocampal connectivity in vivo and potentially enable early diagnosis of AD.Methods
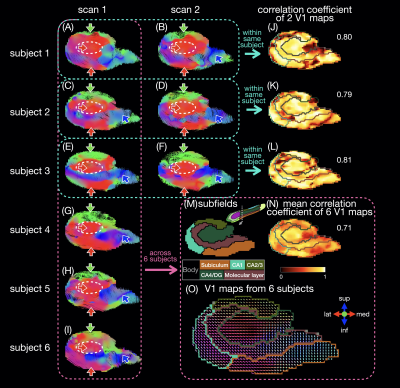
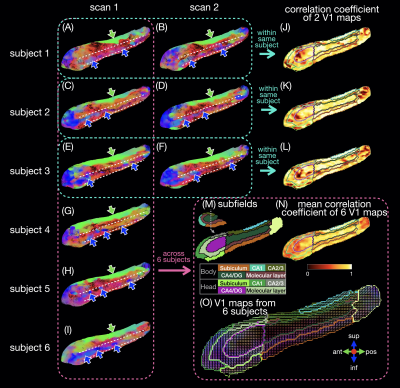
Six healthy subjects (29±7 years old) were scanned on a 3T scanner (GE Premier UHP, 113 mT/m gradients), with three subjects scanned twice on different days. Whole-brain DTI data were acquired with a 2-band, 4-shot EPI sequence (axial, TR/TE 8530/63 ms, 0.9 mm isotropic, 4 b0 + 38 b 800 s/mm2, 23:53 min), reconstructed with MB-MUSE6,7, and preprocessed8-15. T1-weighted anatomical images (MPRAGE, 0.9 mm isotropic) were registered to the DTI images and used along with the b0 images to generate a hippocampal mask and hippocampal subfields with FreeSurfer.16A unilateral hippocampus template was generated using MRtrix3 population_template15 with multi-contrast images from the nine scans (b0 images, mean of all diffusion-weighted images, hippocampal mask, and potential maps17,18). Each DTI scan was registered to the template with a deformable registration. Maps of the principal eigenvector (V1) were generated and reoriented based on the Jacobian matrix at each voxel15. In each voxel, a correlation coefficient was calculated as the dot product between two V1 vectors from repeated scans of the same subject or as the mean dot product between two V1 vectors from all available pairs of subjects. Intra-hippocampal fiber tracts (tensor deterministic, length: 1-100 mm, number: 100k) and connectomes were generated with MRtrix315 without scaling by fiber length or subfield volume.
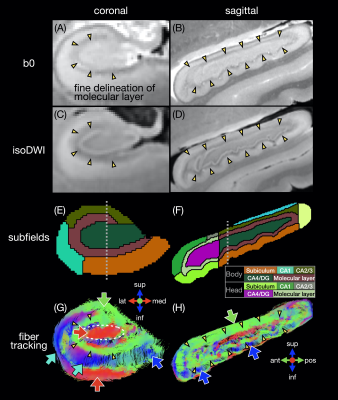
A human temporal lobe specimen was fixed and scanned at 7.1T with a 3D DTI sequence (TR/TE 100/17 ms, 0.2 mm isotropic, 3 b0 + 31 b 1500 s/mm2, 21h25), as described in19. The ex vivo hippocampus was registered to the in vivo template with a rigid registration. The hippocampal mask and subfields were manually drawn to match the FreeSurfer segmentation16. Fiber tracts and the connectome were generated as described above.
Results and Discussion
The in vivo fiber tracking (Figs.1-2) shows characteristic fiber orientations (A-I) in different hippocampal subfields (M) that are generally consistent between repeated scans and across all subjects: right/left (red arrows) in the middle of the CA4/dentate gyrus subfield (ovals) and the inferior part of the subiculum; anterior/posterior (green arrows) in CA2/CA3; superior/inferior (blue arrows) in the medial and inferior parts (in the coronal and sagittal views, respectively) of the molecular layer and subiculum. The correlation coefficients between two V1 vectors from repeated scans (J-L) and averaged across all pairs of subjects (N) are generally high. The V1 vectors (O) are also generally well aligned across all subjects.The ex vivo fiber tracking (Fig.3) shares similar features (G,H; red, green, blue arrows) with the in vivo results, although superior/inferior tracts in the inferior-lateral part of the molecular layer and CA1 (cyan arrows) are not found across all in vivo results. Additionally, there is a clear delineation of anterior/posterior tracts in the molecular layer (C-shaped in the coronal view; arrowheads in A-D,G,H) not seen in vivo, likely because of the higher resolution in the ex vivo DTI scan. The fiber orientation in the molecular layer was also anterior/posterior in a previous ex vivo study3, but was found to be more variable in another study depending on the sample orientation4.
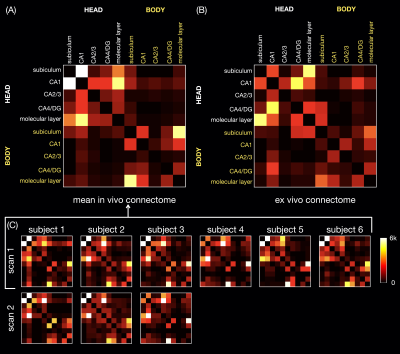
The in vivo and ex vivo connectomes (Fig.4) show more connectivity within the head than within the body of the hippocampus, as shown in a previous ex vivo study3. The in vivo connectomes are generally consistent between repeated scans and across all subjects (A,C) and show the highest connectivity between CA1 and the subiculum or molecular layer in the head and between the subiculum and molecular layer in the body, which are also strong connections in the ex vivo connectome (B). The latter shows the highest connectivity between the subiculum and molecular layer and between CA1 and the CA4/dentate gyrus subfield in the head, which are also strong connections in the in vivo connectomes. In the previous ex vivo study, the connectome3 showed the highest connectivity between the molecular layer and CA1, possibly because of the different subfield segmentation, but this connection is also strong in our in vivo and ex vivo connectomes.
Conclusion
The intra-hippocampal fiber orientations and connectivity across different subfields can be assessed in vivo, which could potentially be used for the diagnosis of neurodegenerative diseases.Acknowledgements
This work was in part supported by the National Institutes of Health (grants R01 EB028644, R01 NS075017, and S10 OD021480).References
[1] Moodley KK, Chan D. The hippocampus in neurodegenerative disease. The hippocampus in clinical neuroscience. 2014;34:95-108.
[2] Maller JJ, Welton T, Middione M, et al. Revealing the hippocampal connectome through super-resolution 1150-direction diffusion MRI. Scientific reports. 2019 Feb 20;9(1):1-3.
[3] Beaujoin J, Palomero-Gallagher N, Boumezbeur F, et al. Post-mortem inference of the human hippocampal connectivity and microstructure using ultra-high field diffusion MRI at 11.7 T. Brain Structure and Function. 2018 Jun;223(5):2157-79.
[4] Ly M, Foley L, Manivannan A, et al. Mesoscale diffusion magnetic resonance imaging of the ex vivo human hippocampus. Human Brain Mapping. 2020 Oct 15;41(15):4200-18.
[5] Wu D, Zhang J. In vivo mapping of macroscopic neuronal projections in the mouse hippocampus using high-resolution diffusion MRI. Neuroimage. 2016 Jan 15;125:84-93.
[6] Chen NK, Guidon A, Chang HC, et al. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013 May 15;72:41-7.
[7] Bruce IP, Chang HC, Petty C, et al. 3D-MB-MUSE: A robust 3D multi-slab, multi-band and multi-shot reconstruction approach for ultrahigh resolution diffusion MRI. Neuroimage. 2017 Oct 1;159:46-56.
[8] Veraart J, Novikov DS, Christiaens D, et al. Denoising of diffusion MRI using random matrix theory. Neuroimage. 2016 Nov 15;142:394-406.
[9] Veraart J, Fieremans E, Novikov DS. Diffusion MRI noise mapping using random matrix theory. Magnetic resonance in medicine. 2016 Nov;76(5):1582-93.
[10] Kellner E, Dhital B, Kiselev VG, et al. Gibbs‐ringing artifact removal based on local subvoxel‐shifts. Magnetic resonance in medicine. 2016 Nov;76(5):1574-81.
[11] Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003 Oct 1;20(2):870-88.
[12] Andersson JL, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. 2016 Jan 15;125:1063-78.
[13] Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001 Jan;20(1):45-57.
[14] Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004 Jan 1;23:S208-19.
[15] Tournier JD, Smith R, Raffelt D, et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. 2019 Nov 15;202:116137.
[16] Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015 Jul 15;115:117-37.
[17] Adler DH, Wisse LE, Ittyerah R, et al. Characterizing the human hippocampus in aging and Alzheimer’s disease using a computational atlas derived from ex vivo MRI and histology. Proceedings of the National Academy of Sciences. 2018 Apr 17;115(16):4252-7.
[18] DeKraker J, Lau JC, Ferko KM, et al. Hippocampal subfields revealed through unfolding and unsupervised clustering of laminar and morphological features in 3D BigBrain. Neuroimage. 2020 Feb 1;206:116328.
[19] Badea A, Wang N, Cofer GP, et al. High resolution magnetic resonance histology of the human brain temporal lobe. Proc ISMRM 25th. 2017;4269.
Figures