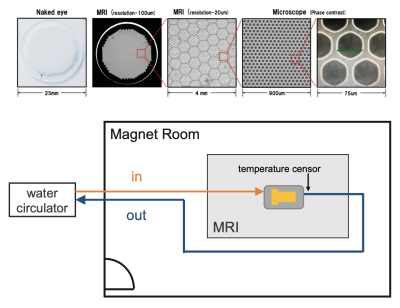
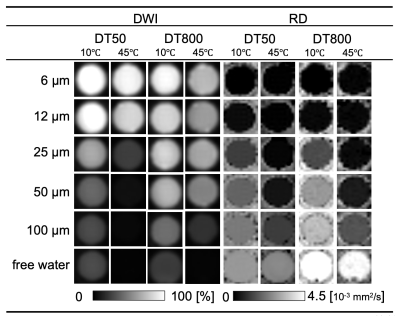
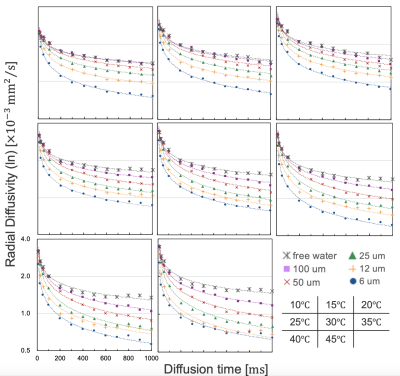
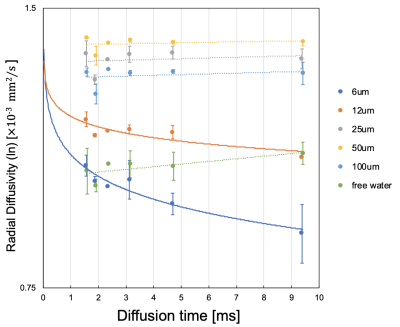
4748
Characteristics of three factors that influence the diffusion signal with PGSTE and OGSE1Tokyo Metropolitan University, Tokyo, Japan, 2RIKEN Center for Brain Science, Saitama, Japan, 3Keio University School of Medicine, Tokyo, Japan, 4The Jikei University School of Medicine, Tokyo, Japan
Synopsis
We investigated the characteristics of diffusion quantification values in a restricted structure with a capillary phantom in PGSTE and OGSE. Smaller structure sizes require shorter diffusion times for structure estimation. In OGSE, we observed a transition of diffusion coefficients under 10 ms for capillary sizes from 6-12 μm, but no differences for those from 25-100 μm. Moreover, it was necessary to consider the temperature dependence of the diffusion measurement at low temperatures. We developed a system to measure the effects on diffusion quantification values at three phases of different temperatures, structure sizes, and diffusion times.
Introduction
Quantitative values in diffusion magnetic resonance imaging (dMRI) can be measured by detecting the displacement of water molecules in diffusion. The diffusion quantitative values can estimate the micron-level structure. Many histological visualization studies have been conducted, such as detection of white matter mutations in rat spinal cord(1) and evaluation of skeletal muscle fiber characteristics(2). It is known that diffusion quantification is diffusion time dependent, and the effects have been reported in cerebral white matter nerves(3) and cortical tissue(4). However, few basic physical data have been reported from experimental investigations using restricted structures, such as capillary phantoms. Although water-molecule diffusion depends on temperature(5), the relationship between temperature dependence and structure size has not been investigated in detail. The study aim was to comprehensively vary the structure size, diffusion time, and temperature to investigate the characteristics of restricted dMRI of a capillary phantom.Methods
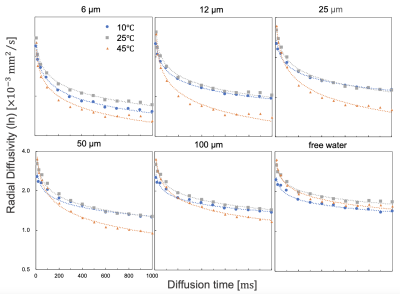
We used phantoms were made of capillary plates, 6, 12, 25, 50, 100 μm (Hamamatsu Photonics, Japan) filled with pure water and vacuum degassed. We performed pulsed-gradient stimulated echo (PGSTE), diffusion time= 12-1000 ms, b-value= 1800 s/mm2, large delta/small delta= 3.6/13.7 ms, TR/TE= 4000/17.97 ms, matrix= 128×128, FOV= 60×60 mm, diffusion directions= 12, number of averages= 1 and oscillating-gradient spin echo (OGSE), sine waveform, number of periods= 6, diffusion time (3/8f)= 1.6–9.4 ms, f= 40–240 Hz, b-value= 1000 s/mm2, gradient strength= 106–640 mT/m, TR/TE= 2500/60.86 ms, matrix= 128×128, FOV= 60×60 mm, diffusion directions= 12, number of averages= 3, temperature= 25 ℃ with on a 9.4 Tesla MRI system with an 86 mm quadrature coil (Bruker Biospin, Ettlingen, Germany).To measure the measurement temperature dependence, we created a constant-temperature measurement system that used a 3D printer. The phantom was set up in the system, and constant-temperature water (10, 15, 20, 25, 30, 35, 40, and 45 ℃) was circulated around the phantom and in the adiabatic tube to measure temperature dependence. We directly monitored the temperature inside the device to maintain the system temperature (Figure 1). We analyzed the diffusion quantification values by calculating the diffusion tensor (Diffusion toolkit). In this study, we used radial diffusivity, with the diffusion direction orthogonal to the running of the glass tube in the capillary phantom. Several regions of interest were set in the phantom, and the diffusion coefficients were acquired for each structure size and temperature. We evaluated the characteristics of the diffusion quantification values for the three factors (temperature, diffusion time, and structure size).
Results
The MRI data from this study was summarized as Figure 2. For large capillary sizes, such as 25–100 μm, larger structure sizes required diffusion times > 100 ms for structure detection (Figure 3). OGSE is able to set a very short diffusion time < 10 ms, which PGSTE cannot achieve, and we confirmed the transition of diffusion coefficients with a slight change in the diffusion time for capillary sizes of 6–12 μm. However, OGSE showed no difference in radial diffusivity for large sizes from 25–100 μm because the diffusion time was too short (Figure 4). To estimate the structure size, we needed to set up an appropriate sequence and diffusion time for each sample. The temperature dependence was stronger for shorter diffusion times and larger structure sizes (Figure 5). Higher temperatures enhance the Brownian motion of water molecules, which increases the diffusion displacement. For small structures, the distance to the wall is short, so water molecules hit the wall quickly and changes with temperature are small. On the other hand, in a larger structure, the distance to the wall is longer, so water molecules take longer to collide with the wall and the number of water molecules that hit the wall depends on the temperature and diffusion time.Discussion
In a typical cell size of 25 μm, at 25 ℃ (room temperature) and 45 ℃ (close to in vivo temperature), the diffusion quantification value decreased by 37 % even with a sufficient diffusion time of 800 ms. Diffusion quantification values deviate from many reported values under low-temperature conditions(6)(7). Therefore, it is necessary to consider the effect of temperature dependence for specimens and phantoms. Water molecules hit the structure wall quickly in smaller structure sizes, and our results showed that smaller structures required shorter diffusion times for structure evaluation. Moreover, OGSE was useful for detecting micron-level structures, such as neurons(8), and PGSTE was effective for measuring larger structures, such as hepatocytes and myocytes, suggesting that it is important to select an appropriate diffusion time. In this study, we used a capillary phantom with a completely restricted structure. However, the water-molecule diffusion in vivo was limited by semipermeable membranes. Hence, we need to estimate water-molecule behavior in vivo more precisely by using a permeabilized phantom and simulation studies.Conclusion
We measured the diffusion time required for structure estimation following the Langevin equation of water-molecule diffusion. The system we developed measured the temperature, structure size, and diffusion time to investigate the characteristics of restricted diffusion in MRI of a capillary phantom.Acknowledgements
This research is partially supported by the program for Brain Mapping by Integrated Neurotechnologies for Disease Studies (Brain/MINDS) from Japan Agency for Medical Research and development (AMED) and by “MRI platform” as a program of Project for Promoting public Utilization of Advanced Research Infrastructure of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank Araki Rikita, Koichiro Takahashi, and Hishinuma Takashi, Bruker Japan, for support with MRI systems.
References
1) Assaf Y, Mayk A, Cohen Y. Displacement imaging of spinal cord using q-space diffusion-weighted MRI. Magn Reson Med, 2000, Nov;44(5):713-22.
2) Hata J, Nakashima D, Tsuji O, et al. Noninvasive technique to evaluate the muscle fiber characteristics using q-space imaging. PLOS ONE, 2019, 14(4): e0214805.
3) C. A. Baron, C. Beaulieu. Oscillating gradient spin-echo (OGSE) diffusion tensor imaging of the human brain. Magnetic Resonance in Medicine, 2014, 72(3), 726–736.
4) N. Pyatigorskaya, D. Le Bihan, O. Reynaud, et al. Relationship between the diffusion time and the diffusion MRI signal observed at 17.2 tesla in the healthy rat brain cortex. Magnetic Resonance in Medicine, 2014, 72(2), 492–500.
5) M. Holz, S. R. Heil, A. Sacco. Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Physical Chemistry Chemical Physics, 2000, 2(20), 4740–4742.
6) S. Kodama, J. Hata, Y. Kanawaku, et al. Determining the effect of water temperature on the T1 and T2 relaxation times of the lung tissue at 9.4 T MRI: A drowning mouse model. Legal Medicine, 2021, 49(November 2020), 101836.
7) Y. Haga, J. Hata, A. Uematsu, et al. MR imaging properties of ex vivo common marmoset brain after formaldehyde fixation. Magnetic Resonance in Medical Sciences, 2019, 18(4), 253–259.
8) B. Siow, I. Drobnjak, A. Ianus, et al. Axon radius estimation with Oscillating Gradient Spin Echo (OGSE) Diffusion MRI. Diffusion Fundamentals, 2013, 18(1), 1–6.
Figures