4746
Exploiting slice-by-slice B1 adjusted adiabatic refocusing RF pulses to improve diffusion weighted imaging at 7T1Melbourne Brain Centre Imaging Unit, The University of Melbourne, Melbourne, Australia, 2Department of Biomedical Engineering, The University of Melbourne, Melbourne, Australia, 3Siemens Healthcare Pty Ltd, Melbourne, Australia, 4Siemens Healthcare Pty Ltd, Brisbane, Australia
Synopsis
The application of diffusion weighted imaging (DWI) at ultra-high field is hampered by severe B1 inhomogeneities leading to signal dropouts in certain parts of the brain. Utilising TR-FOCI pulses in a twice-refocussed spin echo DWI sequence can effectively recover signal in these areas, albeit at the cost of increased SAR. Maintaining this enhanced B1 homogeneity, this work demonstrates a ~30% reduction in SAR based on a slice-by-slice RF amplitude optimization.
Introduction
Ultra-high field imaging offers an increased signal-to-noise ratio to benefit imaging techniques like diffusion-weighted imaging (DWI), which is an indispensable tool to study microstructure in the human brain. B1 inhomogeneities associated with higher field strengths remain a challenge and lead to signal dropouts at the base of the brain that impede the application of DWI at 7T1. Using TR-FOCI pulses2 in a twice refocussed spin echo (TRSE) DWI3 sequence can effectively mitigate the signal dropouts by improving the refocussing performance, albeit at a significantly increased specific absorption rate (SAR)4.This work extends the concept and reduces the SAR demands of the TRSE DWI sequence by leveraging the known varying B1 profiles across slices by introducing a slice-by-slice RF amplitude optimisation for the TR-FOCI refocusing pulses6. The concept offers a ~30% SAR reduction while maintaining the same refocussing performance.
Methods
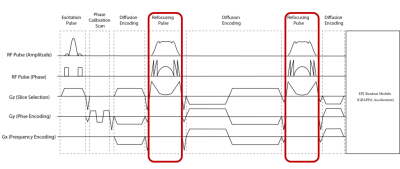
A conventional twice refocussed 2D EPI DWI sequence was adapted by replacing the SLR-optimised refocussing pulses with optimised TR-FOCI pulses (Figure 1) whose B1 amplitude is adjustable on a slice-by-slice basis. The employed TR-FOCI pulses were optimised using a genetic algorithm and hill-climbing5 and were designed to achieve a slice thickness of 2mm with a pulse length of 10ms.The concept was evaluated with measurements on a cylindrical phantom and a healthy volunteer (male, 40 yrs) with written informed consent obtained. All measurements were performed on an investigational 7T whole body MRI scanner (MAGNETOM 7T plus, Siemens Healthcare, Erlangen, Germany) using an 1Tx-32Rx head-coil (Nova Medical Inc., MA, USA).
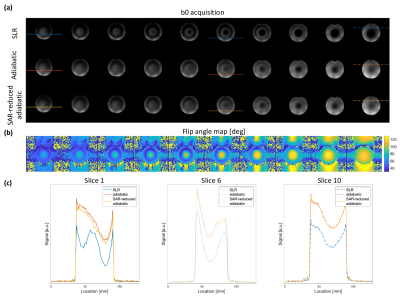
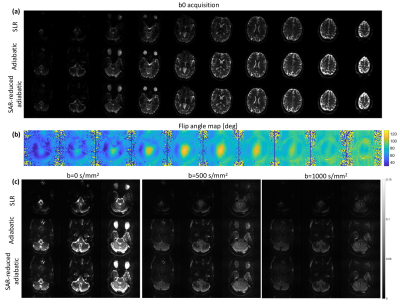
Comparing acquisitions between SLR-optimised, TR FOCI and SAR-reduced TR-FOCI implementations, three image sets were acquired with: 10 slices, 500% distance factor, 230 mm FOV, 1.8 mm isotropic resolution, 2mm slice thickness, 2056 Hz/px readout bandwidth, GRAPPA = 3, TR = 15000 ms, b-values = [0, 500, 1000] s/mm2. For highest SNR, each acquisition was measured at the shortest achievable TE. Given the longer pulse length of the TR-FOCI pulses, TE was slightly longer at 74ms vs 64ms for the SLR-optimised product implementation.
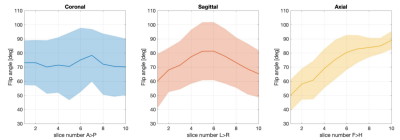
TurboFLASH-based B1 maps were acquired at the same slice locations to inform the slice-by-slice TR-FOCI SAR-optimisation. In vivo B1-maps were acquired in axial, sagittal and coronal directions to illustrate the imaging direction with the best SAR reduction potential from a slice-by-slice adjustment. We know that the mean and standard deviation of flip angles in an axial, sagittal and coronal slice orientations vary differently. While flip angles show a greater spread within sagittal and coronal slices, the intra-slice variation is less in axial slices (Figure 3). This means that the axial direction has a clearer separation between low B1 performance in inferior and good B1 performance in superior slices. Axial orientation thus offers the greatest potential for slice-by-slice SAR-optimised RF scaling.
Results
Phantom b0 images highlight the improved refocussing performance of adiabatic TR-FOCI pulses compared with the SLR-optimised implementation (Figure 2a). Signal level and homogeneity are improved across each slice. The variation in B1 profiles across the object (Figure 2b) allowed for a 28% SAR reduction when scaling the TR-FOCI B1 amplitude on a slice-by-slice basis, leveraging the better B1 characteristics towards the centre of the coil (right-hand-side of displayed slices, Figure 2). Importantly, there is no visual difference between SAR-reduced and the original TR-FOCI acquisitions. This is further illustrated by line profiles across different slices and horizontal positions in the imaged phantom, showing only negligible signal differences (Figure 2c).The observed signal improvements in the phantom experiments are also observed in in vivo b0 acquisitions (Figure 4a). Adiabatic TR-FOCI based refocussing shows enhanced signal across the brain, particularly around the cerebellum, an area that is particularly affected by B1-related signal dropouts. Leveraging the varying B1 characteristics of each axial slice (Figure 4b) facilitated a 31% reduction in SAR without any visual loss in performance. Focussing on the lower three slices covering the cerebellum shows that the signal improvements are furthermore maintained across b-values with stronger and more homogeneous signal for b=500 mm/s2 and b=1000 mm/s2 acquisitions (Figure 4c).
Discussion and Conclusion
While originally designed for inversions, TR-FOCI pulses can be used for refocussing in a twice-refocussed experiment due to the double plane rotation that leads to a complete refocussing of the signal. As in the case of an inversion, pulse performance remains stable when sufficient adiabaticity is reached4,6. With differing B1 conditions along the coil and the imaged object, this saturation effect on pulse performance motivates the slice-by-slice optimisation to find the lowest required TR-FOCI B1 amplitude for each slice and reduce the overall SAR burden.This study focused on axial orientation, however, oblique slice orientations might offer even better conditions for SAR optimisation. Furthermore, B1-shimming using parallel transmit (pTx) capabilities may offer an additional means to further reduce the TR-FOCI B1 amplitudes required to minimise SAR in DWI and opportunities to refine the pulse design of the excitation pulse to provide other avenues for SAR reduction or homogeneity performance that would be multiplicative to this work’s improvements.
In conclusion we found that the TR-FOCI pulses can reduce DWI signal dropouts in twice-refocussed experiments and that a slice-by-slice pulse optimisation offers an approximately 30% power reduction somewhat mitigating the increased SAR burden.
Acknowledgements
We would like to thank Neuroscience Victoria for supporting the fellowship that facilitated this work. Furthermore, we would like to thank Markus Bath and Shahrokh Abbasi-Rad from the University of Queensland who helped with the sequence implementation and advised on the sequence setup.References
[1] Ibrahim TS, Lee R, Abduljalil AM, Baertlein BA, Robitaille PM. Dielectric resonances and B 1 field inhomogeneity in UHFMRI: computational analysis and experimental findings. Magnetic resonance imaging. 2001;19(2):219-26.
[2] Shen J, Chen Z, Yang J. FOCI with reduced RF power requirements. Journal of Magnetic Resonance Imaging 2004;20:531–537.
[3] Reese TG, Heid O, Weisskoff R, Wedeen VJ. Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magnetic Resonance in Medicine. 2003;49(1):177-82.
[4] Abbasi-Rad S, Cloos M, Jin J, O'Brien K, Barth M. B1+ inhomogeneity mitigation using adiabatic refocusing RF pulses for diffusion weighted imaging at 7T. Proceedings of the Annual Meeting of the International Society of Magnetic Resonance in Medicine. 2021;29:0916.
[5] Hurley AC, Al‐Radaideh A, Bai L, Aickelin U, Coxon R, Glover P, et al. Tailored RF pulse for magnetization inversion at ultrahigh field. Magnetic Resonance in Medicine. 2010;63(1):51-8.
[6] Abbasi-Rad, S, O’Brien, K, Kelly, S, et al. Improving FLAIR SAR efficiency at 7T by adaptive tailoring of adiabatic pulse power through deep learning. Magnetic Resonance in Medicine. 2021;85:2462– 2476.
Figures