4745
Distortion-Free DWI using PSF-EPI at High-Performance 0.5T MRI1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Marvel Stone Healthcare Co., Ltd., Wuxi, China
Synopsis
Low-field MR systems with state-of-the-art hardware may improve the accessibility of MRI. DWI is valuable for diagnosing diseases, while it requires multiple averages at low-field MRI considering SNR. Given similar acquisition time, multi-shot EPI DWI can be considered alternatively. Particularly, the tilted-CAIPI accelerated multi-shot PSF-EPI has been proposed in recent years to achieve fast distortion-free DWI. This study tested the feasibility of PSF-EPI on a high-performance 0.5T MR scanner, and proposed a method for further noise reduction using B0 field information. The results show that PSF-EPI eliminates distortions and improves structure fidelity at tissue boundaries compared with interleaved EPI.
Introduction
The development of low-field MR systems (e.g., 0.5T), with state-of-the-art hardware, has the potential to contribute to an overall increase in the accessibility of MRI in clinical practice, due to the less cost of equipment purchase, installation, maintenance and operation 1. DWI has been proved valuable in diseases such as stroke, brain tumor, and cerebral infarction 2-5. Single-shot echo planar imaging (ssEPI) is the mostly adopted technique for DWI due to its high acquisition efficiency and insensitivity to bulk motion. However, ssEPI suffers from T2* blurring and geometric distortions even at low field. Due to the intrinsically low SNR of low-field MR, multiple averages are necessary for ssEPI. Given similar acquisition time, multi-shot acquisition, such as interleaved EPI (iEPI) and readout-segmented EPI (rs-EPI) 6,7 can be considered alternatively, with the benefits of reduced B0 field inhomogeneity-induced distortions and T2* blurring. Particularly, a novel multi-shot acquisition method, the tilted-CAIPI accelerated point-spread-function encoded EPI (PSF-EPI), has been proposed in recent years to achieve fast distortion- and blurring-free DWI 8-11.PSF-EPI adopts an additional phase encoding gradient before the EPI readout of a 2D ssEPI sequence 8, generating a pseudo 3D k-space consisting of kx (readout), ky (phase encoding, PE) and ks (PSF-encoding) dimensions. 3D iFT of the pseudo 3D k-space forms an image space containing x, y, z dimensions. The distortion-free images can be obtained through sum-of-square (SOS) along the y dimension12. In the presence of noise (Figure 1B, yellow circle), the SOS operation will heighten the noise floor of the final image. One strategy is to filter the PSF line in the s-y plane 13, yet the filter method should be carefully considered.
This study tested the feasibility of fast distortion-free PSF-EPI DWI on a 0.5T MRI scanner. A noise reduction method, which is based on PSF line filtering using B0 field information, was further proposed to reduce the noise floor. iEPI DWI was also acquired for comparison.
Methods
This study was approved by the local Institutional Review Board and written informed consents were obtained from healthy volunteers. Whole-brain data were acquired on a Marvel Stone 0.5T scanner (Marvel Stone Healthcare Co., Ltd., China), using an 8-channel head coil. The maximum gradient strength and slew rate are 40 mT/m and 200 T/m/s, respectively.Data Acquisition and Reconstruction
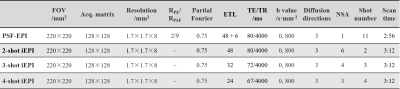
The specific parameters of the PSF-EPI and iEPI sequences are shown in Table 1.
For PSF-EPI, the tilted-CAIPI kernel 8 in the ky-ks plane was adopted. 6 extra ky lines were acquired for self-navigated inter-shot phase correction of the diffusion data. The under-sampled data were reconstructed by PE- and PSF-GRAPPA 8.
For iEPI, the data were reconstructed by iterative POCSMUSE 14.
B0-Informed Noise Floor Reduction of PSF
In PSF-EPI, the actual PSF line deviates from the ideal diagonal PSF line in areas with B0 field inhomogeneity 13, manifesting as pixel shift along the y-direction for each s position (Figure 2A). A diagonal filter (Figure 1C) may mask out the signal in some areas with large shift large field inhomogeneity on the PSF line, resulting in signal loss of the final image. Therefore, an adaptive filter should be used to match the actual PSF line.
For each s-position, the pixel shift distance along the y-direction (Figure 2A, black arrows) can be calculated from the field map
$$d(x,y)=\Delta f(x,y) \cdot Ny \cdot ESP$$
where d(x,y) is the calculated shift map (Figure 2B); ∆f(x,y) is the B0 field inhomogeneity in hertz; Ny is the image matrix size in the PE direction. Then the actual y-position for each x- and s- coordinate can be obtained. The actual y-positions are selected and form a mask with a finite width (Figure 1D) to select the actual PSF line, thereby eliminating the noise outside the mask. A thinner filter width is expected to eliminate more unexpected signals. This process is repeatedly performed along the s-direction and at every frequency encoding step (x-direction). In PSF-EPI, 2D iFT of the kx-ks plane forms an image for each ky, yielding multi-echo image series along ky. Nine ky echoes were used for field map estimation here.
Results and Discussion
Figure 3 shows the PSF-EPI b=0 and mean DWI images, without filtering and with different filtering methods, respectively. The proposed adaptive filter effectively reduced the noise floor compared to the images without filtering. When using a small width, the adaptive filter can preserve the signal in areas with large field inhomogeneity, while the diagonal filter is likely to lose signal in such areas due to improper filtering of the PSF line (Figure 3, yellow arrows).Figure 4 shows the b=0 and mean DWI images of PSF-EPI and iEPI using different combinations of shots and averages. Compared to iEPI, PSF-EPI effectively removes the residual distortions (Figure 4A and C, arrows), and shows improved structure fidelity at tissue boundaries (Figure 4B and D, arrows).
Conclusion
This study demonstrated the feasibility of fast distortion-free PSF-EPI DWI on a high-performance 0.5T MR system, and further reduced its noise floor using an adaptive filter for PSF lines using B0 field information. The superiority of PSF-EPI over interleaved EPI was also demonstrated.Acknowledgements
No acknowledgement found.References
1. Wardlaw JM, Brindle W, Casado AM, et al. A systematic review of the utility of 1.5 versus 3 Tesla magnetic resonance brain imaging in clinical practice and research. Eur Radiol 2012;22(11):2295-2303.
2. Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19(6):1061-1066.
3. Fiebach J, Jansen O, Schellinger P, et al. Comparison of CT with diffusion-weighted MRI in patients with hyperacute stroke. Neuroradiology 2001;43(8):628-632.
4. Kono K, Inoue Y, Nakayama K, et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001;22(6):1081-1088.
5. Toyoda K, Kitai S, Ida M, Suga S, Aoyagi Y, Fukuda K. Usefulness of high-b-value diffusion-weighted imaging in acute cerebral infarction. Eur Radiol 2007;17(5):1212-1220.
6. Butts K, de Crespigny A, Pauly JM, Moseley M. Diffusion-weighted interleaved echo-planar imaging with a pair of orthogonal navigator echoes. Magn Reson Med 1996;35(5):763-770.
7. Porter DA, Heidemann RM. High resolution diffusion-weighted imaging using readout-segmented echo-planar imaging, parallel imaging and a two-dimensional navigator-based reacquisition. Magn Reson Med 2009;62(2):468-475.
8. Dong Z, Wang F, Reese TG, et al. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magn Reson Med 2019;81(1):377-392.
9. Hu Z, Wang Y, Dong Z, Guo H. Water/fat separation for distortion-free EPI with point spread function encoding. Magn Reson Med 2019;82(1):251-262.
10. Li G, Wang Y, Shang Y, Qiao Y, Guo H. Cholesteatomas Detection using PSF-encoded EPI DWI. In Proceedings of the 27th Annual Meeting of ISMRM. Montreal, Canada, 2019. p. 0350.
11. Zhang J, Wang Y, Xiong Y, Guo H. Distortion-free diffusion-weighted imaging of the eyeballs and the optic nerves using PSF-EPI. In Proceedings of the 27th Annual Meeting of ISMRM. Montreal, Canada, 2019. p. 0345.
12. In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. Neuroimage 2017;148:20-30.
13. Oh SH, Chung JY, In MH, et al. Distortion correction in EPI at ultra-high-field MRI using PSF mapping with optimal combination of shift detection dimension. Magn Reson Med 2012;68(4):1239-1246.
14. Zhang Z, Zhang B, Li M, et al. Multishot cartesian turbo spin-echo diffusion imaging using iterative POCSMUSE Reconstruction. J Magn Reson Imaging 2017;46(1):167-174.
Figures
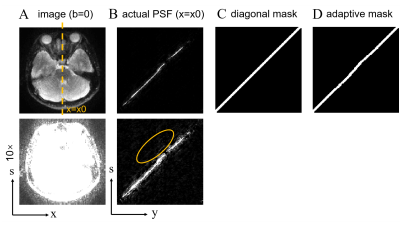
Figure 1. (A) A distortion-free b=0 image after sum-of-square (SOS) along the s-dimension. (B) One PSF line in the s-y plane, for the x=x0 position. (C) A diagonal mask to filter the PSF line, mask width=5. (D) An adaptive mask that suits the actual PSF line trajectory based on local B0 field information, mask width=5.
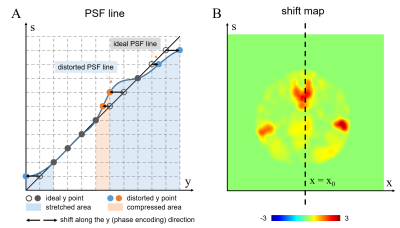
Figure 2. (A) Illustration of pixel shift along the y-direction (phase encoding) of each s point, in the presence of B0 field inhomogeneity. (B) The pixel shift map calculated from the B0 field map.
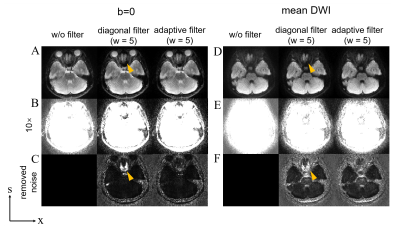
Figure 3. The PSF-EPI b=0 (A, B) and mean DWI images (D, E), without filtering and with different filtering methods, respectively. (C) and (F) show the 10× noise map removed from the un-filtered image. The yellow arrows indicate that the diagonal filter with a width=5 results in signal loss in the area with large B0 field inhomogeneity, since some signals on the PSF line were masked out.
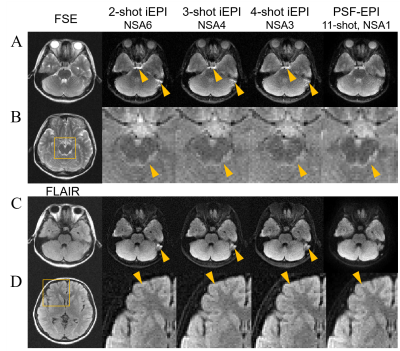
Figure 4. Comparison of the b=0 and mean DWI images between interleaved EPI (iEPI) and PSF-EPI. FSE and FLAIR images serve as the anatomical reference.