4744
Metallic Artifact Suppression in DWI Using Efficient 2D Imaging1Center for Biomedical Imaging Research, Tsinghua University, Beijing, China, 2Philips Healthcare, Beijing, China, 3MR Clinical Science, Philips Healthcare, Suzhou, China
Synopsis
Diffusion-weighted imaging (DWI) near metal remains a technical challenge although several 3D multispectral imaging methods have achieved effective metallic artifacts correction in anatomical MRI. Additionally, considering scan time and scan flexibility, 2D multi-slice acquisition approaches are typically used in DWI. In this study, we adopted 2D Point-Spread-Function Encoded EPI to generate a series of echo-shifted in-plane distortion-corrected images and introduced an off-resonance frequency-informed reconstruction framework to further reduce metallic artifacts in DWI by through-slice signal registration. The efficacy of this method was validated in phantom experiments, in-vivo brain DTI and spinal cord DWI.
Introduction
Metal-induced susceptibility artifacts can significantly impede MRI examinations for patients with implanted devices. In the past decade, the problem of metallic artifacts has been effectively resolved by three-dimensional multispectral imaging (3D MSI) methods, including SEMAC 1, MAVRIC 2 and MAVRIC SL 3.Despite the success of 3D MSI in anatomical MRI, these methods are not appropriate for DWI due to long scan time and inflexibility even with under-sampling strategies 4. Thus, metallic artifacts correction in DWI remains a substantial challenge given that the commonly-used EPI for DWI is highly sensitive to susceptibility inhomogeneity. Additionally, the prolonged scan time and reduced SNR of DWI are also important considerations. Therefore, 2D imaging approaches are desirable for DWI near metal. A previously proposed 2D FSE-based PROPELLER DWI 5 demonstrates reduced metallic artifacts in body DWI at a cost of acquisition time and slice coverage. Recently, a novel 2D multi-slice acquisition approach, SEMSI 11, provides off-resonance correction near metal for anatomical imaging by taking advantage of shifted spin-echo readouts. This offers new insights into metallic artifacts correction in 2D imaging, especially for DWI.
In this study, we aimed to explore a tailored 2D imaging technique for metallic artifacts reduction in DWI. Here we adopted a multi-shot EPI technique, Point-Spread-Function Encoded EPI (PSF-EPI), and proposed a reconstruction framework to improve artifacts correction. The efficacy of this method was validated in phantom imaging, in vivo brain DTI and spinal cord DWI with metal implanted.
Methods
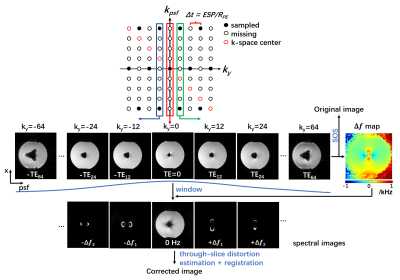
Basic principles for off-resonance estimation in PSF-EPIIn PSF-EPI, an additional phase encoding gradient is exerted to the phase encoding direction before the EPI readout 6-9. This provides a pseudo 3D k-space consisting of $$$k_x$$$, $$$k_y$$$ , $$$k_{psf}$$$ dimensions. Since the signals at given $$$k_y$$$ step are acquired at the same echo time, the $$$x$$$-$$$psf$$$ images along $$$k_y$$$ dimension can be regarded as a series of GRE images with shifted echo times. This enables the estimation of off-resonance frequency. Here, we also used tilted-CAIPI 10 under-sampling along with Parallel imaging for high acceleration.
Image Reconstruction
The under-sampled k-space was firstly restored by GRAPPA-like interpolation with tilted kernels 10. Then, the recovered k-space is used to generate echo-shifted image series by 2D iFFT. As shown in Fig. 1, the signal void can vary greatly with different shifted echo times TEi due to the significantly increased off-resonance induced by metal. To reduce this echo-time dependent signal cancellation, a weighting window was used before further correction. As TEi of each x-psf image is known, the off-resonance frequency ($$$Δf$$$) map can be estimated. Here, we used a graph-cut approach from the Fat-water Toolbox 12. Then the $$$Δf$$$ maps were incorporated into the reconstruction, following by sum-of-square combination along $$$k_y$$$ to generate an in-plane off-resonance corrected image. This image is further corrected for through-slice signal displacement according to $$$Δf/BW_{RF}*slice thickness$$$ . The flowchart for reconstruction is shown in Fig. 1.
Data Acquisition
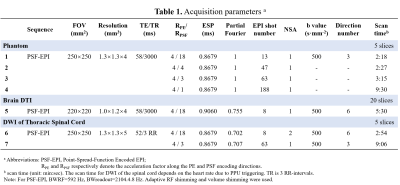
The MRI scans were performed on a Philips 3T Ingenia CX scanner (Philips Healthcare, Best, The Netherlands). This study was approved by the Institutional Review Board and written informed consent was obtained from each participant. SEMAC-VAT images were acquired as anatomical reference in the phantom experiment with in-plane resolution=0.89×0.89 mm2, SENSE=4, SEMAC factor=25, scan time = 33min 16s. Additionally, T2W TSE images with and without SPIR were also acquired for comparison. PSF-EPI with tilted-CAIPI at different acceleration rates were performed to evaluate the efficacy on artifacts correction. Detailed scan parameters are shown in Table. 1.
Results and Discussion
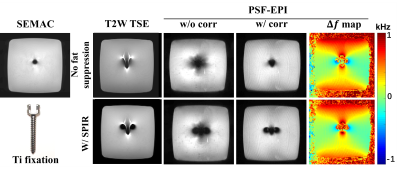
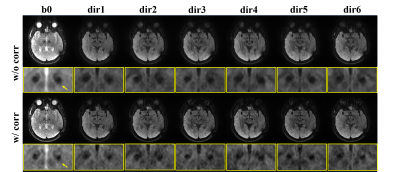
Fig. 2 shows the comparison of PSF-EPI images with or without correction in the phantom experiment. As shown, using SPIR might increase metallic artifacts due to undesired signal suppression of off-resonances near metal. The $$$Δf$$$ maps from PSF-EPI with and without SPIR are similar, both indicating a nearly 1 kHz frequency offset around metal. The corrected PSF-image demonstrates distinctly improved signal void compared with that without correction, which is more similar with the SEMAC reference. Note the ripple-pattern ringing is probably caused by the windowing, which needs further investigation.Fig. 3 compares the PSF-EPI images with and without correction of a patient with implanted DBS electrodes. Both b0 and DWI images after correction demonstrated smaller regions of signal loss although with slightly reduced SNR.
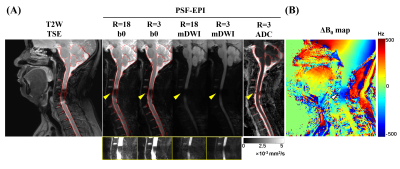
Fig. 4 demonstrates the results of a post-operative patient with anterior Titanium plates and screws in the cervical spine. Severe signal loss and pile-up are shown in T2W TSE. The metal-induced signal loss (yellow arrows) is partly restored with lower acceleration rate in PSF-EPI. However, the signal loss cannot be completely restored due to the partial volume effects from the thick slice. But the ADC map obtained from PSF-EPI (RPSF=3) data almost fully recover the signals near metal, indicating effective signal recovery although with low magnitude.
Conclusion
In this study, we introduced a reconstruction framework for metallic artifacts reduction in DWI using a 2D multi-slice acquisition. The results of both the phantom experiment and in vivo studies show the promise of our method for effective off-resonance correction near metal in DWI. Higher BWRF and BWreadout will be used to test on larger implants. Optimization of reconstruction framework for highly accelerated PSF-EPI will also be explored.Acknowledgements
No acknowledgement found.References
1. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice encoding for metal artifact correction in MRI. Magnetic Resonance in Medicine 2009;62(1):66-76.
2. Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magnetic Resonance in Medicine 2009;61(2):381-390.
3. Koch KM, Brau AC, Chen W, et al. Imaging near metal with a MAVRIC-SEMAC hybrid. Magnetic Resonance in Medicine 2011;65(1):71-82.
4. Hargreaves BA, Taviani V, Litwiller DV, Yoon D. 2D multi-spectral imaging for fast MRI near metal. Magnetic Resonance in Medicine 2018;79(2):968-973.
5. Koch KM, Bhave S, Gaddipati A, et al. Multispectral diffusion-weighted imaging near metal implants. Magnetic Resonance in Medicine 2018;79(2):987-993.
6. In M-H, Tan ET, Trzasko JD, et al. Distortion-free imaging: A double encoding method (DIADEM) combined with multiband imaging for rapid distortion-free high-resolution diffusion imaging on a compact 3T with high-performance gradients. Journal of Magnetic Resonance Imaging 2020;51(1):296-310.
7. In MH, Cho S, Shu Y, et al. Correction of metal-induced susceptibility artifacts for functional MRI during deep brain stimulation. Neuroimage 2017;158:26-36.
8. Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med 2004;52(5):1156-1166.
9. Oh SH, Chung JY, In MH, et al. Distortion correction in EPI at ultra-high-field MRI using PSF mapping with optimal combination of shift detection dimension. Magn Reson Med 2012;68(4):1239-1246.
10. Dong Z, Wang F, Reese TG, et al. Tilted-CAIPI for highly accelerated distortion-free EPI with point spread function (PSF) encoding. Magnetic Resonance in Medicine 2019;81(1):377-392.
11. Daehyun Y, Lee P et al. Spectrally-encoded multi-spectral imaging (SEMSI) for off-resonance correction near metallic implants. Proc. of Intl. Soc. Mag.Reson. Med. 29 (2021). p0669.
12. ISMRM fat-water separation workshop 2012.‘http://www.ismrm.org/workshops/FatWater12/’
Figures