4741
Improved MR Temperature Imaging at 0.5T with Multi-echo Thermometry1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2Marvel Stone Healthcare Co., Ltd., Wuxi, China
Synopsis
Low field MR-guided thermotherapy can provide some key advantages over the high-field alternative, including reduced cost, decreased susceptibility artifacts, and improved safety of interventional devices. However, both the accuracy and the speed of PRF temperature measurement suffer at the low field due to the reduced SNR, limited receive channels, and declined temperature-induced phase changes, making it unreliable for clinical MRgLITT treatments. In this study, we demonstrate that the multi-echo thermometry together with the view-sharing acceleration can be utilized to achieve high-quality PRF thermometry at 0.5T with satisfactory temperature measurement precision and temporal resolution.
Introduction
MR-guided laser interstitial thermal therapy (MRgLITT) has been applied as a minimally invasive treatment in neurosurgery. Recent research1 has shown the great potential of low field MR systems (such as 0.55T) on MR-guided interventions and thermotherapies, benefiting from reduced cost, declined interventional device heating and decreased susceptibility artifacts. However, the precision of the proton resonance frequency (PRF) shift-based thermometry2 can be deteriorated at low-field due to low SNR3,4, hindering its clinical reliability for LITT temperature monitoring. Additionally, sampling acceleration is needed in MRgLITT to ensure enough temporal resolution, yet maintaining SNR in the meantime has to be considered.This study aims to improve the temperature precision at low-field using multi-echo GRE and to accelerate the acquisition using a view-sharing approach5-9 without sacrificing SNR. The performance of this method was validated on a 0.5T scanner.
Theory and Methods
The influence of B0 Decrease on PRF ThermometryPRF thermometry is challenging at 0.5T due to the decrease of the magnetic field B0.
- The accuracy of temperature measurement drops dramatically due to the B0 decrease. The uncertainty of temperature measurement at 0.5T is 20 times higher than that at 1.5T according to (1)3:
$$\delta T=\sqrt{2}/(2\pi\cdot\gamma\cdot B_0\cdot\alpha\cdot TE\cdot SNR),\ \ SNR\propto B_0^{7/4}\ \ \ (1)$$
- The sensitivity of temperature measurement is declined at 0.5T because temperature-induced phase difference is inversely proportional to B0 according to (2)2 :
$$\triangle \phi = \alpha\triangle T\cdot\gamma B_0\cdot TE\ \ \ (2)$$
Multi-echo Thermometry
Longer TE can provide better temperature precision and sensitivity according to Eq. (1) and (2) in GRE-based thermometry. Therefore, multi-echo thermometry10,11 with its ability to use a wide range of TEs without increasing scan time is well suited at low fields. A bipolar GRE sequence12 was applied to achieve higher SNR efficiency.
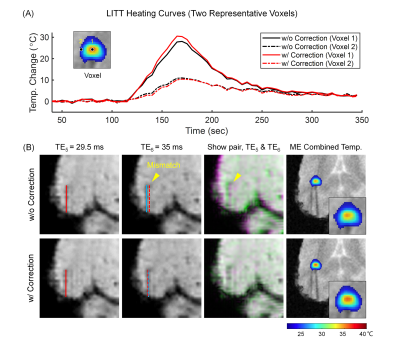
- Echo Misregistration Correction: Since the receiver bandwidth is kept low to ensure sufficient SNR in this study, field inhomogeneity and eddy current-induced misregistration between even and odd echoes is corrected13 with an estimated B0 field map from multi-echo data.
- Echo Combination: The measured temperature maps are combined into a single estimate using a tSNR optimal weighted echo combination approach14, whereby the weights are the production of image magnitude and TE.
View-sharing Acceleration
The view-sharing-based approach, with its ability to achieve fast reconstruction, and to preserve accelerated image SNR without the need for multiple receive channels, is beneficial for the low field. So this study adopted the view-sharing acceleration strategy and investigated its feasibility.
Experiments
All experiments were performed in a 0.5T MRI scanner (Marvelstone, Wuxi, China) with an eight-channel receive coil. Temperature data were acquired by a multi-echo bipolar GRE with the following parameters: flip angle = 30°, TE = 7.5~40.5ms, nTE / delta TE = 7 / 5.5ms, TR = 138ms, matrix = 108×110, FOV = 220×220 mm2, slice thickness = 5mm, 3 slices (no gap), sampling bandwidth (BW) = 27.8 kHz, view-sharing acceleration = 3 (variable density), temporal resolution = 5 sec/volume.
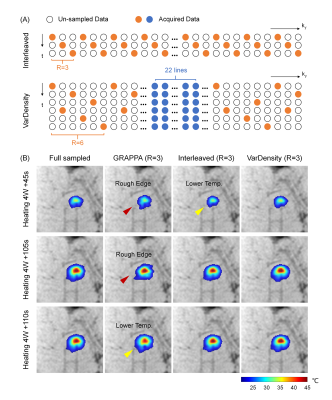
- Simulation Experiments: Fully sampled data were first acquired on pork tissues using the sequence mentioned above but without acceleration (temporal resolution=12 sec/volume, heat at 4W for 120s). Two different k-t undersampling patterns (Fig.1A) were tested. A sliding window-based algorithm5, which recovered undersampled k-space using previous time frames, was implemented for reconstruction. The reconstructed temperature maps were compared to those reconstructed using GRAPPA.
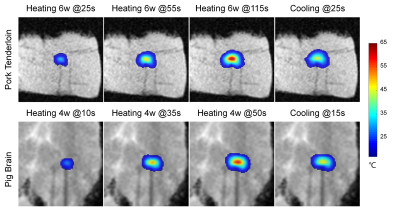
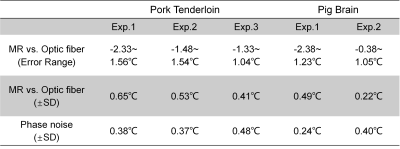
- In Vitro Tissue Heating Experiments: In vitro MRgLITT experiments (MR-Guided Laser Ablation System, Sinovation Medical, Beijing, China) were carried out in pork tenderloin samples (N=3, heat at 6W for the 60s, wait for 60s, then heat for another 60s) and pig brain tissues (N=2, heat at 4W for 50s) separately. An MR-compatible fiber optical sensor was inserted into the tissue to obtain ground truth. Temperature uncertainty was calculated as the standard deviation (SD) of the temperature difference between MR and fiber-optic measurements.
Results and Discussion
Multi-echo ThermometryAs illustrated in Fig. 2, the image misregistration between odd and even echoes can lead to wrong temperature measurements (e.g. reduced temperatures at the heating center). Therefore, echo misregistration correction is necessary for bipolar multi-echo thermometry, especially when using low readout bandwidth (greater SNR and also greater echo mismatch).
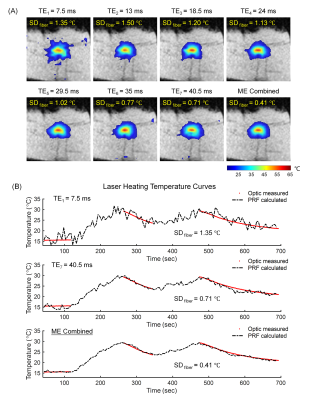
Fig. 3 demonstrates that the precision of temperature measurement can be substantially improved after echo combination when compared with single-echo measurements. Therefore, multi-echo thermometry is highly recommended for low-field PRF measurement.
View-sharing Acceleration
Simulation experiments show finer temperature maps with lower errors in the 3-fold view-sharing acquisition (VarDensity, Fig. 1B). GRAPPA reconstructed images suffer from SNR decrease which can lead to increased temperature uncertainty. View-sharing acceleration (Interleaved) fails at rapid temperature rise (Fig. 1B, first row) probably because the reconstruction uses too much temperature data from previous time frames.
Temperature Measurement Accuracy
Multi-echo thermometry with view-sharing acceleration (3-fold, VarDensity) results in high-quality temperature maps (Fig. 4) and excellent precision with the average temperature uncertainty of less than 0.8°C (Tab. 1) in in vitro heating experiments.
Conclusion
We have shown experimentally that multi-echo thermometry with view-sharing acceleration can achieve high-quality PRF thermometry at 0.5T. The measured temperature uncertainty is within a clinically acceptable threshold (<3°C). Still, it needs further investigation on whether the view-sharing acceleration is acceptable from a clinical perspective, as the temperatures may change more complicatedly during treatment.Acknowledgements
No acknowledgement found.References
1. Campbell-Washburn AE, Ramasawmy R, Restivo MC, et al. Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology 2019;293(2):384-393.
2. Ishihara Y, Calderon A, Watanabe H, et al. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med 1995;34(6):814-823.
3. Botnar RM, Steiner P, Dubno B, Erhart P, von Schulthess GK, Debatin JF. Temperature quantification using the proton frequency shift technique: In vitro and in vivo validation in an open 0.5 tesla interventional MR scanner during RF ablation. Journal of Magnetic Resonance Imaging 2001;13(3):437-444.
4. Waqas Majeed AJK, Sunil Patil, Henrik Odéen, John Roberts, Florian Maier, Dennis L. Parker, and Himanshu Bhat. Feasibility of Magnetic Resonance Thermometry at 0.55T. Proc Intl Soc Mag Reson Med 29 (ISMRM & SMRT Virtual Conference & Exhibition), 2021. p.
5. Riederer SJ, Tasciyan T, Farzaneh F, Lee JN, Wright RC, Herfkens RJ. MR fluoroscopy: technical feasibility. Magn Reson Med 1988;8(1):1-15.
6. Jones RA, Haraldseth O, Muller TB, Rinck PA, Oksendal AN. K-space substitution: a novel dynamic imaging technique. Magn Reson Med 1993;29(6):830-834.
7. van Vaals JJ, Brummer ME, Dixon WT, et al. "Keyhole" method for accelerating imaging of contrast agent uptake. J Magn Reson Imaging 1993;3(4):671-675.
8. Doyle M, Walsh EG, Blackwell GG, Pohost GM. Block regional interpolation scheme for k-space (BRISK): a rapid cardiac imaging technique. Magn Reson Med 1995;33(2):163-170.
9. Han Y, Mun C. Evaluation of the keyhole technique applied to the proton resonance frequency method for magnetic resonance temperature imaging. J Magn Reson Imaging 2011;34(5):1231-1239. 10. Marx M, Pauly KB. Improved MRI Thermometry with Multiple-Echo Spirals. Magn Reson Med 2016;76(3):747-756.
11. Svedin BT, Payne A, Bolster BD, Parker DL. Multiecho pseudo-golden angle stack of stars thermometry with high spatial and temporal resolution using k-space weighted image contrast. Magn Reson Med 2018;79(3):1407-1419.
12. Wieben O LJ, Mansson S, Hennig J. Multi-echo balanced SSFP imaging for iterative Dixon reconstruction. Proceedings of the 13th Annual Meeting of ISMRM Miami Beach, 2005. p.
13. Lu W, Yu H, Shimakawa A, Alley M, Reeder SB, Hargreaves BA. Water-fat separation with bipolar multiecho sequences. Magn Reson Med 2008;60(1):198-209.
14. Madore B, Panych LP, Mei CS, Yuan J, Chu RX. Multipathway Sequences for MR Thermometry. Magn Reson Med 2011;66(3):658-668.
Figures