4740
QSM from the raw phase using an end-to-end neural network1School of ITEE, University of Queensland, Brisbane, Australia, 2Queensland Brain Institute, University of Queensland, Brisbane, Australia, 3Centre for Advanced Imaging, University of Queensland, Brisbane, Australia, 4Departments of Radiology and Clinical Neurosciences, University of Calgary, Calgray, AB, Canada
Synopsis
Deep learning frameworks are emerging methods for solving QSM problems these days. However, most previous deep neural networks designed for QSM requires phase unwrapping and background field removal preprocessing procedures. This work presents a novel end-to-end network, namely Lap-Unet, for instant QSM and tissue field mapping from the raw phase in a single run. Comparative results find that the proposed method resulted in more accurate and robust reconstructions than previously established single- and multi-step methods, particularly for QSM of intracranial hemorrhages, which has been challenging due to fast signal decays.
Introduction
QSM is an MRI phase-based postprocessing technique that can extract valuable susceptibility distribution in the human brain in a non-invasive manner. However, traditional QSM reconstruction pipelines involve several non-trivial intermediate processing steps, including phase unwrapping, background removal, and ill-posed dipole inversion. These sequential steps not only amplify the noise and errors but increase the reconstruction time. This study presents deep neural network-based instant quantitative field and susceptibility mapping (i.e., iQFM and iQSM) methods by designing a novel Laplacian filtering-based layer (Lap-Layer). In addition, simulation and in vivo experiments were conducted to compare iQFM and iQSM with multiple established QSM pipelines.Methods
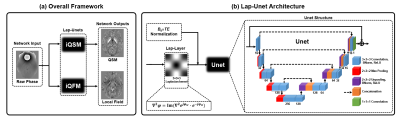
Lap-UnetAs shown in Fig. 1, the proposed iQFM and iQSM methods are based on a novel Lap-Unet architecture, consisting of (i) a novel Lap-Layer and (ii) a 3D residual Unet commonly used in previous deep learning QSM methods. The Lap-Layer is designed to process the wrapped raw phase and implemented according to Eq. (1):
Lap-Layer(φw) = ∇2sin(φw) • cos(φw) - ∇2cos(φw) • sin(φw), (1)
where ∇2 is the 27-point stencil discrete Laplacian operator; φw is the raw phase (i.e., input of the network). The Unet comprises 19 convolutional layers, 18 batch normalisation layers, 4 max-pooling, 4 unpooling layers, 4 concatenation layers, and a final skip connection.
Training data preparation
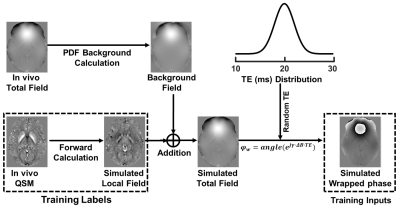
A total of 13824 small susceptibility map patches (size: 643) were randomly cropped from 96 full-size brain volumes. Then, for each QSM patch, a simulated hemorrhage or a calcification source were super-positioned onto the original patch at a predefined probability (40%). Next, the training data were prepared using the pipeline shown in Fig. 2. The iQSM network was trained with simulated wrapped phase as inputs and susceptibility as labels, while iQFM was trained with wrapped phase as inputs and local field as labels.
Network training
All network parameters were initialised with normally distributed random numbers of 0 mean and 0.01 standard deviation. Then, using Adam optimiser, all networks were trained for 100 epochs (20 hours) on two Nvidia Tesla V100 GPU. The batch size was 32, and the learning rate was 10-3, 10-4, and 10-5 for the first 40 epochs, 40-80 epochs, and the final 20 epochs. MSE was used as the loss function.
Validation on simulated and in vivo datasets
In this work, the proposed iQFM and iQSM were compared with several established methods, including single-step TGV-QSM1, best path2 and laplacian unwrapping3, RESHARP4, LBV5, PDF6, SHARQnet7 background removal; iLSQR8, QSMnet+9, AutoQSM10 based dipole inversion. Except for AutoQSM, which was based on Laplacian unwrapping results, all other multi-step methods started with the best path unwrapping results.
Four brain data (two healthy and two pathological) were simulated based on two COSMOS data using the pipeline described in Fig. 2 to validate the Lap-Layer's effectiveness and quantitatively compare iQFM and iQSM with multiple established methods. Besides, one in vivo data from a patient with intracranial hemorrhage was acquired at 3T with 1 mm isotropic resolution to compare iQSM with previous QSM methods.
Results
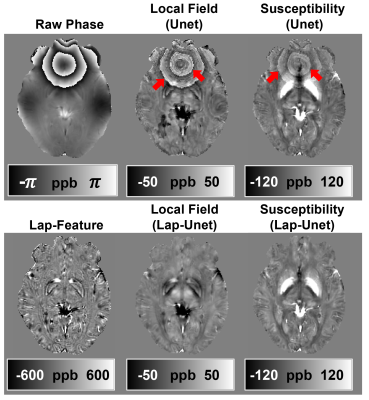
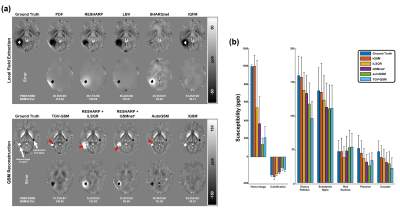
Figure 3 demonstrates that the networks trained without the Lap-Layer (i.e., Unet based methods) led to severe artifacts on the phase wraps. By contrast, the proposed iQFM and iQSM successfully eliminated these artifacts. This comparison confirms that the novel Lap-Layer can effectively remove the phase wraps from the raw wrapped phase images, enabling successful training of the following Unet.Figure 4 compares the reconstruction results of different QFM and QSM methods. The iQFM and iQSM are the only two methods with no substantial artifacts (red arrows), resulting in the highest PSNR and SSIM. As shown in the bar graph in Fig. 4(b), iQSM also led to the most accurate susceptibility measurements in the hemorrhage, calcification, and deep grey matter regions.
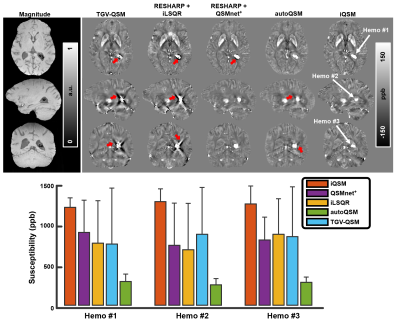
Different QSM reconstruction methods were compared on the in vivo brain data with intracranial hemorrhage in Fig. 5. The proposed iQSM method produced the most visually appealing susceptibility maps with clear delineations of hemorrhages and minimal streaking artifacts, while severe artifacts were exhibited in the results of other methods. The bar graph in Fig. 5 also confirmed that iQSM showed the highest hemorrhage susceptibility measures, while iLSQR, QSMnet+, and autoQSM underestimate hemorrhage susceptibilities, which is consistent with the results on the simulated data (i.e., Fig. 4).
Discussion and Conclusion
This study proposed a Lap-Unet based method for instant tissue field mapping and QSM reconstruction from raw phase images. The new iQFM and iQSM networks obtained more accurate results than several established pipelines, with substantially suppressed artifacts around high susceptibility sources, such as intracranial hemorrhage.Acknowledgements
HS acknowledges support from the Australian Research Council (DE210101297).
GBP acknowledges support from the Canadian Institutes for Health Research (CIHR FDN-143290) and the Campus Alberta Innovates Program (RC-12-003).
References
1. Langkammer, C., Bredies, K., Poser, B. A., Barth, M., Reishofer, G., Fan, A. P., . . . Ropele, S. (2015). Fast quantitative susceptibility mapping using 3D EPI and total generalised variation. Neuroimage, 111, 622-630.2.
2. Abdul-Rahman, H., Arevalillo-Herraez, M., Gdeisat, M., Burton, D., Lalor, M., Lilley, F., . . . Qudeisat, M. (2009). Robust three-dimensional best-path phase-unwrapping algorithm that avoids singularity loops. Appl Opt, 48(23), 4582-4596.3.
3. Li, W., Avram, A. V., Wu, B., Xiao, X., & Liu, C. (2014). Integrated Laplacian-based phase unwrapping and background phase removal for quantitative susceptibility mapping. NMR Biomed, 27(2), 219-227.4.
4. Sun, H. F., & Wilman, A. H. (2014). Background Field Removal Using Spherical Mean Value Filtering and Tikhonov Regularization. Magnetic Resonance in Medicine, 71(3), 1151-1157.5.
5. Zhou, D., Liu, T., Spincemaille, P., & Wang, Y. (2014). Background field removal by solving the Laplacian boundary value problem. NMR Biomed, 27(3), 312-319.6. 6
6. Liu, T., Khalidov, I., de Rochefort, L., Spincemaille, P., Liu, J., Tsiouris, A. J., & Wang, Y. (2011). A novel background field removal method for MRI using projection onto dipole fields (PDF). NMR Biomed, 24(9), 1129-1136.7.
7. Bollmann, S., Kristensen, M. H., Larsen, M. S., Olsen, M. V., Pedersen, M. J., Ostergaard, L. R., . . . Barth, M. (2019). SHARQnet - Sophisticated harmonic artifact reduction in quantitative susceptibility mapping using a deep convolutional neural network. Z Med Phys, 29(2), 139-149.8.
8. Li, W., Wang, N., Yu, F., Han, H., Cao, W., Romero, R., . . . Liu, C. (2015). A method for estimating and removing streaking artifacts in quantitative susceptibility mapping. Neuroimage, 108, 111-122.9.
9. Jung, W., Yoon, J., Ji, S., Choi, J. Y., Kim, J. M., Nam, Y., . . . Lee, J. (2020). Exploring linearity of deep neural network trained QSM: QSMnet+. Neuroimage, 211, 116619.10.
10. Wei, H., Cao, S., Zhang, Y., Guan, X., Yan, F., Yeom, K. W., & Liu, C. (2019). Learning-based single-step quantitative susceptibility mapping reconstruction without brain extraction. Neuroimage, 202, 116064.
Figures