4733
The Development of Hollow Gd2O3 Nanospheres for T1 Contrast Agent1Nantong University, Nantong, China, 2Guangzhou First People's Hospital, Guangzhou, China, 3Philips Healthcare China, Guangzhou, China
Synopsis
Gd2O3 hollow nanospheres (HNS) with tuneable size and shell thickness was prepared. The thinnest shell of 30 nm Gd2O3 HNS achieved is 2.9 nm. This ultra-thin Gd2O3 HNS show effective T1 relaxivity in MRI with the highest r1 value of 3.77 mM-1s-1. Furthermore, the Gd2O3 HNS indicate strong chemical and physical stability which will minimize the toxicity caused by released free Gd3+ ions. The monodispersed and tuneable particle size will also facilitate the particle surface modification and blood circulation time design in bio-applications.
Synopsis
In this study, we have synthesized Gd2O3 hollow nanospheres (HNS) with tuneable size and shell thickness through SiO2 nanoparticles sacrificing the template method. The obtained Gd2O3 HNS are highly uniform in both size and thickness. The thinnest shell of 30 nm Gd2O3 HNS achieved is 2.9 nm. It is the smallest and thinnest Gd2O3 HNS that has been reported. This ultra-thin Gd2O3 HNS show effective T1 relaxivity in MRI with the highest r1 value of 3.77 mM-1s-1. Furthermore, the Gd2O3 HNS indicate strong chemical and physical stability which will minimize the toxicity caused by released free Gd3+ ions. The monodispersed and tuneable particle size will also facilitate the particle surface modification and blood circulation time design in bio-applications.Introduction
Gd2O3 nanomaterials with the highly exposed surface are proved to be effective magnetic resonance imaging (MRI) T1 contrast agents. Most research efforts are focusing on ultra-small Gd2O3 nanoparticles [1,2], while sub-100 nm hollow Gd2O3 nanospheres with the ultra-thin shell are another strategy that can be more advantageous to prevent fast renal clearance and enhance cancer-targeted accumulation. Although there have been many reported methods for the synthesis of hollow nanospheres of various materials, the synthesis of Gd2O3 with an ultra-thin shell has not been achieved previously.Methods
We have synthesized Gd2O3 hollow nanospheres with tuneable size and shell thickness using different sizes of SiO2 nanoparticles as templates. The crystalline Gd2O3 ensures high biological and chemical stability. The extremely thin shell provides maximum surface gadolinium which contributes to an improved T1 contrast effect. The monodispersed nanoparticles with tuneable size facilitate the design and engineering of blood circulation time and clearance path.Results
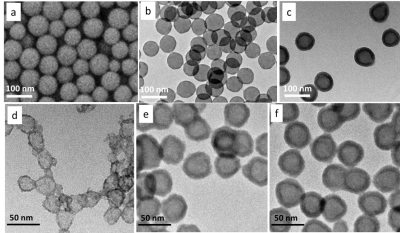
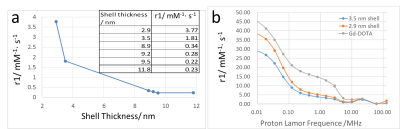
Gd2O3 HNS with different sizes and shell thickness were synthesized using different sizes of SiO2 nanoparticles as templates. The morphologies were examined by TEM as in Fig. 1. The chemical stability was also tested against simulated biological fluid and pH buffer solutions ranging from 3 to 11 with the presence of EDTA on a mechanical shaker for 4 weeks. No released Gd3+ was detected by ICP measurement of the dialysis solutions. T1 relaxivity of 30 nm Gd2O3 HNS with different shell thickness was measured. Fig. 2a shows the plot of r1 value vs. the shell thickness of 30 nm Gd2O3 HNS. With a slight increase of shell thickness from 2.9 nm to 3.5 nm, the r1 value drops dramatically from 3.77 mM-1s-1 to 1.81 mM-1s-1. NMRD profile was also measured to understand the water diffusion within the hollow structure.Discussion
The relaxivity is the sum of these two contributions. But in the case of nanoparticle CAs, the contribution of OS is negligible especially at high fields. Due to macromolecular weight of Gd-DOTA and nanosize of Gd2O3 HNS, all their rotational dynamics are quite slow which eventually influences the relaxivity. Thus, their NMRD curves are highly field dependant according to Solomon-Bloembergen-Morgan theory. The Gd2O3 HNS are mesoporous because of the etching process during the synthesis. The mean pore diameter of 2.9 nm thick Gd2O3 HNS is measured to be 5.8 nm by BET. Thus, water molecules can freely diffuse in and out the cavity of Gd2O3 HNS. However, slow exchange condition is likely to occur and the relaxivity is restricted by relatively long mean residence lifetime τM, which is more dominant at higher fields [3-6].Conclusion
This ultra-thin Gd2O3 HNS show effective T1 relaxivity in MRI with the highest r1 value of 3.77 mM-1s-1. Furthermore, the Gd2O3 HNS indicate strong chemical and physical stability which will minimize the toxicity caused by released free Gd3+ ions. The monodispersed and tuneable particle size will also facilitate the particle surface modification and blood circulation time design in bio-applications.Acknowledgements
No acknowledgement found.References
[1] J.-L. Bridot, A.-C. Faure, S. Laurent, C. Rivière, C. Billotey, B. Hiba, M. Janier, V. Josserand, L. J.-L. Coll, Elst. Vander, R. Muller, S. Roux, P. Perriat, O. Tillement, Hybrid gadolinium oxide nanoparticles: Multimodal contrast agents for in vivo imaging, J. Am. Chem. Soc. 129 (2007) 5076-5084.
[2] Y. Hou, R. Qiao, F. Fang, X. Wang, C. Dong, K. Liu, C. Liu, Z. Liu, H. Lei, F. Wang, M. Gao, NaGdF4 nanoparticle-based molecular probes for magnetic resonance imaging of intraperitoneal tumor xenografts in vivo, Acs Nano. 7 (2012) 330-338.
[3] M. Botta, L. Tei, Relaxivity Enhancement in macromolecular and nanosized GdIII-based mri contrast agents, Eur. J. Inorg. Chem. 12 (2012) 1945-1960.
[4] F. Carniato, L. Tei, M. Botta, Gd-Based Mesoporous Silica Nanoparticles as MRI Probes, Eur. J. Inorg. Chem. 46 (2018) 4936-4954.
[5] F. Carniato, G. Gatti, 1H NMR relaxometric analysis of paramagnetic Gd2O3:Yb nanoparticles functionalized with citrate groups, Inorganics 7 (2019) 34-43.
[6] F. Carniato, M. Muñoz-Úbeda, L. Tei, M. Botta, Selective functionalization of mesoporous silica nanoparticles with ibuprofen and Gd(III) chelates: a new probe for potential theranostic applications, Dalton Trans. 44 (2015) 17927-17931.
Figures