4732
Pseudo-3D whole-brain ultra-thin-slice diffusion-weighted imaging of the brain utilizing deep learning constrained Compressed SENSE1Philips Japan, Tokyo, Japan, 2Tokyo Metropolitan Police Hospital, Tokyo, Japan, 3Philips Healthcare, Best, Netherlands
Synopsis
Deep learning constrained Compressed SENSE reconstruction (CS-AI) achieves significant improvement of image quality of whole-brain, ultra-thin-slice, high-resolution pseudo-3D diffusion imaging using single-shot EPI or single-shot TSE acquisition, compared with conventional SENSE and CS DWI.
PURPOSE
Typical clinical brain diffusion-weighted imaging based on Echo Planar Imaging (DW-EPI) has limited spatial resolution compared to other routine neuro images due to its high sensitivity to B0 inhomogeneities. DW-EPI with smaller voxel size causes further image distortion. Sensitivity encoding (SENSE) helps to reduce the voxel size without increasing image distortion, but it often suffers from increased noise-like artifacts on the center of the images due to the high geometry factor1,2. It has been shown recently that DW-EPI with Compressed SENSE enables pseudo-3D (2D multi-slice acquisition with ultra-thin-slice thickness) whole-brain diffusion imaging with voxel size of 1.15 mm3 in a feasible scan time3. Alternatively, single-shot turbo spin-echo (TSE) DWI is a distortion-free imaging approach but it has also limited spatial resolution due to its low SNR.Recently, CS-AI, integrating artificial intelligence (AI) into the Compressed SENSE reconstruction, based on Adaptive-CS-Net4,5, has been introduced. We hypothesize that the CS-AI reconstruction will further improve the SNR of both DW-EPI and DW-TSE with high spatial resolution and ultra-thin-slice pseudo-3D acquisition. In this study, we attempt to utilize the CS-AI framework for optimizing the pseudo-3D whole-brain diffusion imaging. The purpose of this study is to demonstrate the feasibility of CS-AI in pseudo-3D DW-EPI and DW-TSE imaging.
MATERIALS AND METHODS
A total of five volunteers/patients were examined on a 3.0T whole-body clinical system (Ingenia Elition X, Philips Healthcare). The study was approved by the local IRB, and written informed consent was obtained from all subjects.CS-AI DWI is based on single-shot DW-EPI acquisition with a regular sampling pattern after which the CS-AI framework is used for reconstruction. The CS-AI model used in this study is the extension of the Adaptive-CS-Net algorithm. In CS-AI, the iterative optimization procedure in the C-SENSE reconstruction chain is unrolled for a fixed number of Unet type of blocks. The model was trained on more than 700,000 images, including 2D and 3D data, and multiple contrasts and anatomical areas.
CS-AI-DWI images were compared with conventional SENSE and CS-DWI images for image quality, especially for the reduction of image noise.Imaging parameters for pseudo-3D EPI-DWI were as follows: axial acquisition, voxel size=1.15mm3, FOV=230*230mm, 93slices, b-value=0 and 1200s/mm2, TR=16196ms, TE=65ms, SENSE/C-SENSE acceleration factor = 4.0, and total acquisition time=5min40s, and imaging parameters for pseudo-3D TSE-DWI were: axial acquisition, voxel size=1.5mm3, FOV=220*220mm, 93slices, b-value=0 and 1200s/mm2, TR=5579ms, TE=80ms, SENSE/C-SENSE acceleration factor = 4.0, and total acquisition time=3min16s.
RESULTS AND DISCUSSION
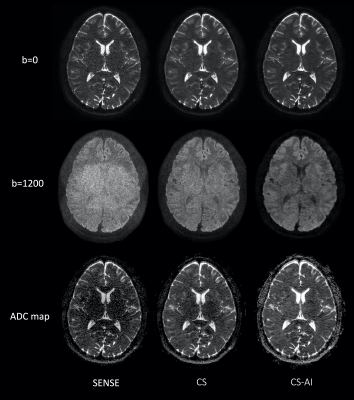
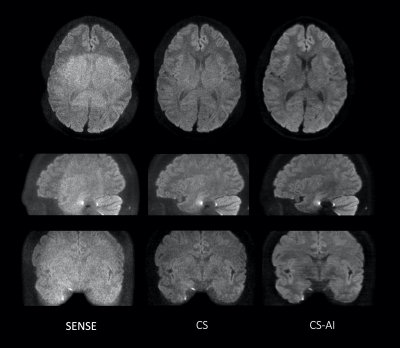
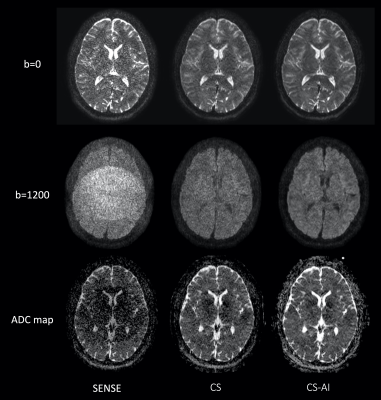
Figure 1 shows representative b=0, 1200s/mm2 images and ADC maps using EPI-DWI with SENSE, CS and CS-AI in a volunteer. CS and CS-AI clearly reduced the noise in the center of the SENSE images. CS-AI further improved the contrast among gray matter, white matter and CSF. Figure 2 shows representative 3mm MPR images with three directions obtained by pseudo-3D EPI-DWI. The p3D-DWI with EPICS achieved high-resolution (1.15 mm3) isotropic DWI within clinically feasible scan time.On the other hand, Figure 3 shows representative b=0, 1200s/mm2 images and ADC maps using TSE-DWI with SENSE, CS and CS-AI in a volunteer and 3mm MPR images with three directions obtained by pseudo-3D TSE-DWI are shown in Figure 4. Similar to EPI-DWI, TSE-DWI with CS-AI clearly reduced the noise and further improved the contrast among gray matter, white matter and CSF.
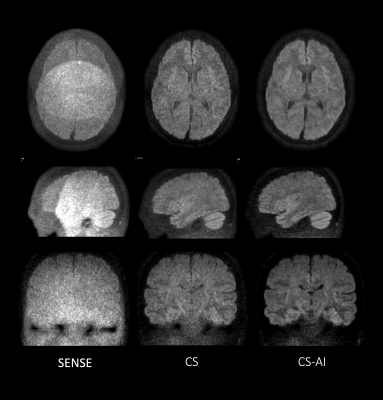
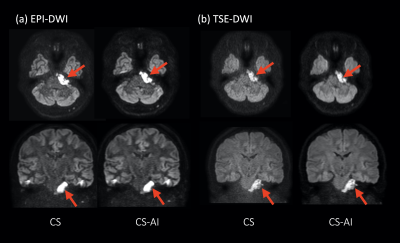
Figure 5 shows representative clinical images of a patient with epidermoid (arrow). CS-AI improved the SNR and contrast especially for the skull base where epidermoid lesion is existing.
CONCLUSION
CS-AI clearly improves the image quality of whole-brain ultra-thin-slice pseudo-3D DWI using single-shot EPI or single-shot TSE acquisition, compared to conventional SENSE and CS DWI. Since this technique is simple and does not require any additional phase correction unlike 3D multi-shot DWI, it would be promising for robust whole-brain high-resolution diffusion imaging within a clinically feasible acquisition time.Acknowledgements
No acknowledgement found.References
1. Patricia N, et al. Parallel Imaging Artifacts in Body Magnetic Resonance Imaging. Can Assoc Radiol J. 2009;60: 91–98.
2. Yanasak NE, et al. MR imaging artifacts and parallel imaging techniques with calibration scanning: a new twist on old problems. Radiographics. 2014;34:532-48.
3. Morita K, et al. Pseudo-3D Diffusion-Weighted Imaging of the Brain using Echo Planar Imaging with Compressed SENSE (EPICS). Proc Intl Soc Mag Reson Med. 2019:3355
4. Pezzotti N, et al. An Adaptive Intelligence Algorithm for Undersampled Knee MRI Reconstruction. IEEE Access. 2020;8:204825-204838.
5. Pezzotti N, et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arxiv. 2019;(NeurIPS).
Figures