4731
High-quality reconstruction of diffusion tensor based on deep learning and multi-slice information sharing1Department of Electronic Science, Fujian Provincial Key Laboratory of Plasma and Magnetic Resonance, Xiamen University, Xiamen, China, 2MSC Clinical & Technical Solutions, Philips Healthcare, Shenzhen, China, 3Department of Magnetic Resonance Imaging, the First Affiliated Hospital of Zhengzhou University, Zhengzhou University, Zhenzhou, China
Synopsis
Diffusion tensor imaging (DTI) requires several diffusion-weighted image (DWI) acquisitions, leading to a long scan time. In this study, a deep-learning model based on multi-slice information sharing was implemented to obtain ultrafast DTI. This method uses the similarity of DWIs among neighboring slices to minimize the requirement of the number of diffusion-encoding directions. The results indicate that the proposed method can reconstruct high-quality diffusion and DTI-derived maps, exceptionally robust to noise in fractional anisotropy mapping even when only 3-direction DWIs.
Introduction
Diffusion tensor imaging (DTI) can quantify tissue microstructure and is useful for tumor classification. The reconstruction of diffusion tensor needs at least seven diffusion-weighted image (DWI) acquisitions since the DTI model has seven free parameters. High-quality DTI usually requires more DWI acquisitions with a series of diffusion directions, which causes a long scan time. Recently, some deep-learning-based methods (e.g., DiffNet1, DeepDTI2, and SuperDTI3) have been proposed to reduce scan time by decreasing the DWI acquisitions, and DTI can keep a high accuracy when only six-direction DWIs are used. In this work, we implemented a deep-learning model combined with multi-slice information sharing to get better performance in modeling DTI, which only used three-direction DWIs every slice, further shortening the scan time. Meanwhile, this method enables higher robustness to noise in fractional anisotropy (FA) mapping by directly modeling the six diffusion tensor parameters.Methods
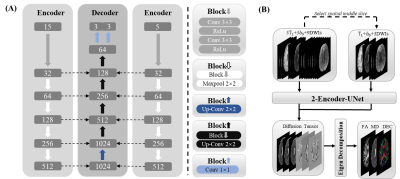
Data acquisition: The data were obtained from the Human Connectome Project (HCP).4 There are 203 subjects in total, 136 for training, 46 for validation, and 21 for testing. T1-weighted images and DWIs with two b values (b = 0, 1000 ms/μm²) were used for this study.Network: The input (Figure 1) of the network has 15 channels from three neighboring slices. Every slice needs a corresponding T1-weighted image for preventing blurring and offering more structural information5, one b = 0 image, and only three b = 1000 ms/μm² DWIs along three diffusion directions for fitting DTI. To share more diffusion information from neighboring slices, the nine DWIs from three neighboring slices are along nine unique diffusion directions, which are the first nine diffusion directions of HCP data for higher robustness to noise6 according to the acquisition protocol of HCP data7. The diffusion direction of DWIs in all slices is a cycle of the first nine diffusion directions of HCP data. The nine DWIs from three neighboring slices were arranged together according to the order of HCP data, so the spatial order of slices was disrupted. The three channels of the T1-weighted images or b = 0 images were arranged according to the current slice order of nine DWIs. The network (Figure 2) was improved from U-Net8 by adding another encoder to make sure the network models the correct slice. Only the diffusion tensor of the middle slice was reconstructed, and the channel position of the middle slice was not fixed. This method mapped the six parameters ($$$D_{xx}$$$, $$$D_{yy}$$$, $$$D_{yy}$$$ , $$$D_{xy}$$$, $$$D_{xz}$$$, $$$D_{yz}$$$) of diffusion tensor ($$$D$$$):
$$D=\begin{bmatrix}D_{xx}&D_{xy}&D_{xz}\\D_{xy}&D_{yy}&D_{yz}\\D_{xz}&D_{yz}&D_{zz}\end{bmatrix}$$
Evaluation: The averaging of 18 b = 0 images and 90 DWIs were used to obtain the ground-truth by conventional model fitting:
$$S_{i}=S_0exp(-bg_{i}^{T}Dg_i)$$
in which $$$S_{i}$$$ and $$$S_0$$$ are the signal intensities of b = 1000ms/μm² and b = 0 images, respectively. And $$$g_i$$$ is the unit direction vector of diffusion.
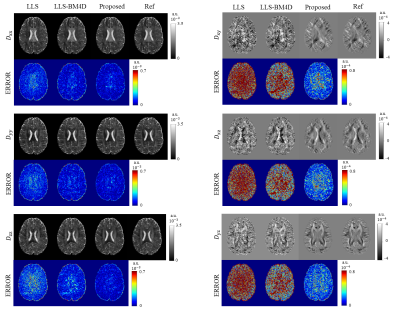
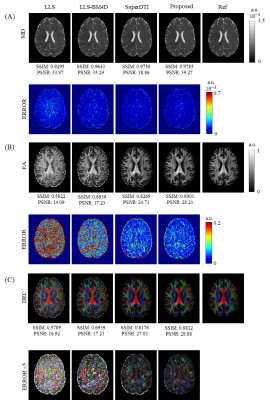
Eigen decomposition was applied to the $$$D$$$ to get three parametric maps, fractional anisotropy (FA), mean diffusivity (MD) and directionally encoded colormap (DEC). The mask was applied to FA to remove the noise around the image boundary, which affected the calculation of the index. For comparison, the results of conventional least-squares fitting (LLS), LLS after block-matching and 4D filtering9 (LLS-BM4D), and SuperDTI3 using 6-direction DWIs were also given. The peak signal-to-noise ratio (PSNR) and structural similarity index (SSIM) were calculated to quantify the reconstruction quality.
Results
Figure 3 shows the reconstruction results of the diffusion tensor. The error images indicate that the six parameter maps reconstructed by our method are very close to the references and obviously better than those obtained by LLS and LLS-BM4D.The reconstructed results of FA, MD and DEC from different methods are given in Figure 4. Our method outperforms other three methods with less diffusion encoding directions used. It should be noted that MD map has higher PSNR and SSIM compared to the FA and DEC maps because the MD map is relatively robust to noise. The erosion algorithm is applied to the FA and DEC references to avoid the influence of noise at tissue boundary on the evaluation of PSNR and SSIM.
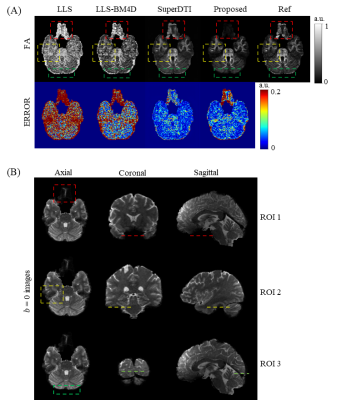
In Figure 5A, a slice with three areas in which obvious noise occurred is shown. Our method illustrates significantly better performance in modeling FA than LLS and LLS-BM4D. In the error images, our method shows similar results to SuperDTI except for the three marked areas. In addition, our method obviously outperforms than all other methods, even the reference at the three ROIs marked in Figure 5A, which are also marked in the b = 0 images from three sections in Figure 5B. Figure 5B indicates that the three ROIs are all at the boundaries of the brain where noise is generated easily during FA modeling. Our method correctly suppresses the noise and enables exact FA mapping, demonstrating its strong robustness to noise in FA mapping.
Discussion and conclusion
In this study, we propose a method for the reconstruction of high-quality diffusion tensor and high-accuracy FA, MD, and DEC maps. The method shows good performance against noise in FA mapping. Meanwhile, the similarity of neighboring slices is utilized so that only 3-direction DWIs are required, which will help to reduce the scan time of DTI.Acknowledgements
This work was supported by the National Natural Science Foundation of China under grant numbers 11775184 and 82071913, and Science and Technology Project of Fujian Province 2019Y0001.
References
1. Aliotta E, Nourzadeh H, Sanders J, Muller D, Ennis DB. Highly accelerated, model-free diffusion tensor MRI reconstruction using neural networks. Med Phys 2019;46(4):1581-1591.
2. Tian Q, Bilgic B, Fan Q, et al. DeepDTI: High-fidelity six-direction diffusion tensor imaging using deep learning. Neuroimage 2020;219:117017.
3. Li H, Liang Z, Zhang C, et al. SuperDTI: Ultrafast DTI and fiber tractography with deep learning. Magn Reson Med 2021;00:1– 14.
4. Van Essen DC, Ugurbil K, Auerbach E, et al. The Human Connectome Project: a data acquisition perspective. Neuroimage 2012;62(4):2222-2231.
5. Golkov V, Dosovitskiy A, Sämann P, et al. Q-space deep learning: twelve-fold shorter and model-free diffusion MRI scans. IEEE Trans Med Imag 2016;35(5):1344-1351.
6. Skare S, Hedehus M, Moseley ME, Li TQ. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J Magn Reson 2000;147(2):340-352.
7. Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 2013;80:125-143.
8. Ronneberger, O., Fischer, P. & Brox, T. U-Net: convolutional networks for biomedical image segmentation. Med. Image Comput. Comput. Assist. Interv 2015;234-241.
9. Dabov K, Foi A, Katkovnik V, Egiazarian K. Image denoising by sparse 3-D transform-domain collaborative filtering. IEEE Trans Image Process 2007;16(8):2080-2095.
Figures
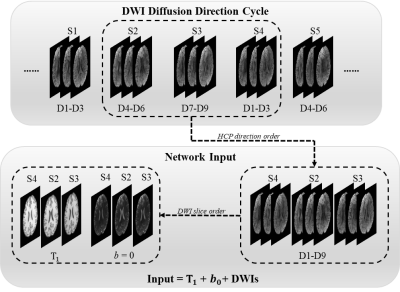
Figure 1. An example of the network input generation. S represents slice, and D represents diffusion direction. The diffusion directions for the brain volume are the cycle of the HCP's first nine directions. The input of the network has three T1 weighted images, three b = 0 images, and nine DWIs from three slices. The DWIs are arranged following the HCP direction order, and the T1 weighted images and b = 0 images are arranged following the slice order of DWI.