4728
Language related cerebrum cerebellum pathway in human: a diffusion imaging‐based tractography study1Department of Neurosurgery, Beijng Tiantan Hospital, Capital Medical University, Beijing, China, 2China National Clinical Research Center for Neurological Diseases, Beijing, China, Beijing, China, 3Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 4University of Chinese Academy of Sciences, Beijing, China, Bei Jing, China
Synopsis
Cerebellum has been proved to play an important role in no motor language function. The aims of this study including: building a new tractography atlas for cerebrum cerebellum pathway base on human connectome project (HCP) datasets which can be used in any study focused on these white matters. Some special tracking strategies were employed in the tracking process. The new tractography atlas was saved including a total of 11 tract templates and performed well in 30 healthy subjects. Both diffusion metrics and shape analysis metrics of these tracts were obtained.
Introduction
Cerebellum has been proved to play an important role in no motor language function of human including word generation, phonologic and semantic processing 1-4. So far, the detailed mechanism of the cerebellum involved in language function has not been fully understood5, 6. It is well accepted that information was transmitted in cerebrum cerebellum circuits. Evidences from neuroanatomy, functional MRI and Viral-tract tracing studies have shown that connections between cerebral cortex and cerebellum exist7-10. However, studies focused on language related cerebrum cerebellum pathway are rarely reported11. The aims of this study including: 1) building a new tractography atlas for cerebrum cerebellum pathway by using the commonly available human connectome project (HCP) datasets; 2) validating the new tractography atlas in healthy subjects; 3) applying the shape analysis of the language related cerebrum cerebellum pathway in the recruited healthy subjects12.Method
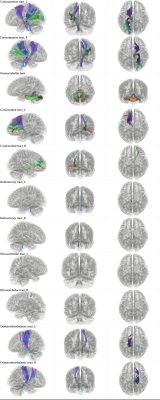
The new tractography atlas was made based on a population averaged template from the HCP-1065(1mm3) datasets13. The 11 tracts (figure 1) were included in cerebrum cerebellum pathway: 1) Corticopontine tract (left and right); 2) Pontocerebellar tract (middle cerebellum peduncle MCP); 3) Corticorubral tract (left and right); 4) Rubroolivary tract (left and right); 5) Olivocerebellar tract (left and right); 6) Dentatorubrothalamic tract (left and right). Diffusion MRI data acquisition was conducted as previously described14. Before tractography, the diffusion MRI data was preprocessed via: data quality checking, artefacts correcting (eddy-current, head movement and field inhomogeneity effects).The DSI_studio software (http://dsi-studio.labsolver.org/) was employed in the tractography atlas making process15. Default tracking parameters were used except seed number was seted at 500000 and the angular threshold was 90 degrees for olivocerebellar tract.
Giving the complex adjacent relationship and high density of nucleus and fibers in related area, special tracking strategies were described below. Due to the perpendicular orientation of the olivocerebellar tract, the angular threshold was set at 90 degrees to obtain this component. In addition, when an olivary nucleus was set as the seed point, the contralateral inferior cerebellum peduncle (ICP) shall be a region of interest (ROI) and the pontine is a region of avoid (ROA) to ensure that fibers originated from olivary nucleus pass through contralateral ICP and finally end at contralateral cerebellum. For corticorubral tract, the red nucleus was set as an additional ROI to narrow down the reticular tracts. For rubroolivary tract, fibers between red nucleus and inferior olivary nucleus was tracked. After these tractography was completed in HCP-1065(1mm3) template, all of them were integrated together as a new tractography atlas for automatic batch fiber tracking. In the validation part, the DTI datasets of 30 healthy subjects were used from our previous published study. The shape analysis of the language related cerebrum cerebellum pathway were conducted using automatic analysis package integrated in the DSI_studio.
Result
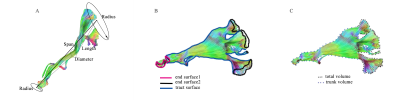
The new tractography atlas was saved including a total of 11 tract templates (figure 3). All 11 tract in 30 healthy subjects were obtained by using the automatic tracking mode. The result of both olivocerebellar tract was discarded they were not consistent with the anatomy facts. So we finally make 9 out of 11tracts of the heathy subjects successfully.The result of tractogryphy including two main parts: 1) diffusion metrics; 2) shape analysis metrics. First, diffusion metrics including: quantitative anisotropy (qa), fractional anisotropy (fa), mean diffusivity (md), axial diffusivity (ad), Radial diffusivity (rd). Second, shape analysis metrics (figure 2) including: mean length, span, curl, elongation, diameter, volume, total surface area, total radius of end regions, total area of end regions, irregularity, area of end region, radius of end region, irregularity of end region. All of these parameters have shown in table 1. On one hand, data clearly quantifies these tracts in diffusion and morphology aspects. On the other hand, the result of MCP need to be treated objectively, when the length and volume were significantly greater than any other tracts due to tractography result. All the quantified result was depend on the tracking result.
Discussion
We firstly have made 11 tract templates from the HCP datasets that perfectly match prior anatomy knowledge. These tract template work well with our health subjects’ dataset except olivocerebellar tract. The diffusion metrics and shape analysis metrics of the language related cerebrum cerebellum pathway were calculated. We try to interpret the result that the olivocerebellar tract originated from inferior olivary nucleus traveling across the middle line to join contralateral ICP. Before that, this tract will cross several fibers. Most importantly, the olivocerebellar tract need to pass through the restiform body where there is spinocerebellar tract, both of them runing lateral to trigeminal nerve branches to enter the ICP. It is difficult to seprate the olivocerebellar tract considering the paraller fibers and complex adjacent relationships. Bedsides, brainstem especially pontine has several disadvantages: a small volume, complex fiber crossing situation and motion artefacts. The imaging quality of in vivo brainstem and cerebellum needs to be improved in order to obtain olivocerebellar tracts successfully. To sum up, this work lay a foundation for further language related white matter analysis about cerebrum cerebellum pathway. As the cerebellum has gradually become a research hotspot, the efferent and afferent projection measurement will definitely become an indispensable part.Acknowledgements
We sincerely thank the prof. Frank Yeh for the discussion about the special tractography strategies. Thanks to Dr. Zhe Zhang for help in data preprocessing and to all participants in this study. This study was funded by the Chinese National Natural Science Foundation (81701088,81870833, 31730039, 61901465), Beijing talents project (2017000021469G211), the Ministry of Science and Technology of China grant (2020AAA0105601, 2019YFA0707103), and the Chinese Academy of Sciences grants (XDB32010300, ZDBS-LY-SM028)References
1. Cho NS, Peck KK, Zhang Z, Holodny AI. Paradoxical Activation in the Cerebellum During Language fMRI in Patients with Brain Tumors: Possible Explanations Based on Neurovascular Uncoupling and Functional Reorganization. Cerebellum 2018;17:286-293.
2. Murdoch BE. The cerebellum and language: historical perspective and review. Cortex 2010;46:858-868.
3. Stoodley C, Schmahmann J. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009;44:489-501.
4. Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. NeuroImage 2000;11:347-357.
5. Diedrichsen J, King M, Hernandez-Castillo C, Sereno M, Ivry RB. Universal Transform or Multiple Functionality? Understanding the Contribution of the Human Cerebellum across Task Domains. Neuron 2019;102:918-928.
6. Marien P, Borgatti R. Language and the cerebellum. Handb Clin Neurol 2018;154:181-202.
7. Leiner HC. Does the Cerebellum Contribute to Mental Skills?. Behavioral Ncuroscicnce 1986.
8. Zhang N, Xia M, Qiu T, et al. Reorganization of cerebro-cerebellar circuit in patients with left hemispheric gliomas involving language network: A combined structural and resting-state functional MRI study. Hum Brain Mapp 2018;39:4802-4819.
9. Verly M, Verhoeven J, Zink I, et al. Altered functional connectivity of the language network in ASD: role of classical language areas and cerebellum. NeuroImage Clinical 2014;4:374-382.
10. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci 2009;32:413-434.
11. Negwer C, Sollmann N, Ille S, et al. Language pathway tracking: comparing nTMS-based DTI fiber tracking with a cubic ROIs-based protocol. J Neurosurg 2017;126:1006-1014.
12. Yeh FC. Shape analysis of the human association pathways. NeuroImage 2020;223:117329.
13. Yeh FC, Panesar S, Fernandes D, et al. Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage 2018;178:57-68.
14. Deng X, Yin H, Zhang Y, et al. Impairment and plasticity of language-related white matter in patients with brain arteriovenous malformations. stroke 2021;accepted.
15. Yeh Fc. DSI Studio. Zenodo 2021;may.
16. Yeh FC, Panesar S, Barrios J, et al. Automatic Removal of False Connections in Diffusion MRI Tractography Using Topology-Informed Pruning (TIP). Neurotherapeutics 2019;16:52-58.
Figures