4727
Multi-shot multi-b-value diffusion-weighted imaging using EPI with keyhole and locally low-rank reconstruction1The Institute of Medical Imaging Technology, School of Biomedical Engineering, Shanghai Jiao Tong University (SJTU), Shanghai, China
Synopsis
A multi-b-value DWI imaging method based on multi-shot EPIK with continuously changing b values and Locally Low-Rank (LLR) constraint is proposed. The multi-shot EPIK acquisition improves resolution of multi-b-value DWI, and acquisition of the central k-space in every b-value bypasses the need for careful initialization. The LLR constrained problem is solved by a newly developed ADMM algorithm with robust performance. Preliminary data in healthy subjects showed that the proposed method outperforms LLR-POCS and SPA-LLR for reconstruction at high undersampling rates.
Introduction
Diffusion Weighted Imaging (DWI) is important for many neuroscientific and clinical applications1. Multi-b-value DWI offers an improved accuracy for Apparent Diffusion Coefficients (ADC) and Diffusion Tensor Imaging (DTI)2, and provides data for IntraVoxel Incoherent Motion (IVIM) mapping3. Typically, DWI is acquired by single-shot EPI, which only samples a limited number of k-space lines causing limited resolution. Recent works have shown that multi-shot EPI based on Locally Low-Rank (LLR) reconstruction is able to achieve high-resolution DWI at a single b-value, provided that the algorithm is initialized by parallel imaging4. However, in multi-b-value imaging, repeated multi-shot EPI over multiple b-values would cause a linearly increasing scan time. Here we propose the use of multi-shot keyhole-EPI (EPIK) with continuously-changing b values and adoption of a different LLR algorithm to achieve robust reconstruction for highly undersampled multi-b-value DWI.Methods
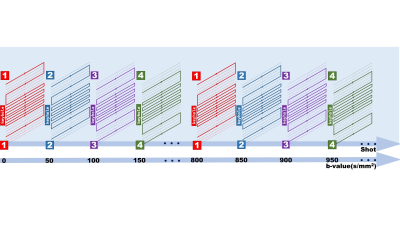
EPI-keyhole was initially developed by Zaitsev et al. to achieve EPI imaging with a high temporal resolution.5 In their work, they showed that EPIK resulted in a distortion less than single-shot EPI provided that a reasonable shimming was performed. In this work, EPIK was combined with interleaved k-space sampling to achieve multi-shot DWI. Furthermore, the b-values are increased at each shot of the EPIK acquisition, facilitating an accelerated multi-shot multi-b-value imaging (Figure 1). The main reason for using EPIK instead of EPI is to bypass the need for a careful initialization of the LLR reconstruction4. This is critical for intra-shot undersampling rates higher than 4, since parallel imaging at higher undersampling rates induces severe noise.Reconstruction of the proposed method involves minimization of the following cost function:
$$\min_x\sum_{b=1}^{Nb}{\frac{1}{2}\lVert E_bFSx_b-y_b \rVert}_{2}^{2}+\lambda \lVert x \rVert _{LLR}\ \left( 1 \right) $$
Where $$$Nb$$$ is the number of b-values,$$$E_b$$$ the sampling operator,$$$F$$$ the DFT matrix,$$$S$$$ the sensitivity maps, $$$x=\left[ \begin{matrix} x_1& \cdots& x_{Nb}\\\end{matrix} \right] ^T$$$ the multi-b-value DWI images,$$$y=\left[ \begin{matrix} y_1& \cdots& y_{Nb}\\\end{matrix} \right] ^T$$$the acquired multi-b-value k‐space signal, $$$\lVert \cdot \rVert _{LLR}$$$ the LLR constraint, which is defined in [9].
To solve Eq 1, we developed an algorithm based on the Alternating Direction Method of Multipliers (ADMM), which is similar to that proposed by Lima da Cruz et al.6 for MR fingerprinting. The LLR-ADMM algorithm supports a densely-overlapped LLR enforcement, which was found by us to generate better reconstruction quality than non-overlapped LLR used by POCS7, SPA-LLR4 and several other groups 8, 9. In this work, we compared our LLR-ADMM algorithm with LLR-POCS and SPA-LLR for reconstruction accuracy.
Imaging was performed in two healthy subjects (age 23±2, 1 male) in a 3T scanner (United Imaging uMR790) with a 32-channel head coil after obtaining written informed consent. A spin-echo single-shot DW EPI sequence was performed with full sampling to acquire 21 b-values ranging from 0 to 1000s/mm2 in an increment of 50 s/mm2. Three DW encoding directions were used. Other parameters were FOV/image size/TR/TE/bandwidth/NEX/flip-angle =240×240mm2/128×128/3000ms/132.3ms/1790Hz/1/90°. Afterwards, a 2-shot with R=1, 2-shot with R=2, and 4-shot with R=2 multi-b-value EPIK sequence with the same resolution and b-values was performed. The intra-shot undersampling rate for these sequences was 2, 4, and 8, respectively, entailing sampling of 76, 50 and 37 k-space lines in each shot, and a corresponding TE of 70.4ms, 69.2ms and 60.1ms, respectively. To validate the method for high-resolution DWI, the 2-shot EPIK with intra-shot R=2 was performed again with 1mm isotropic resolution and TE=100.4ms.
Results
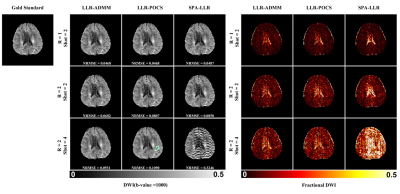
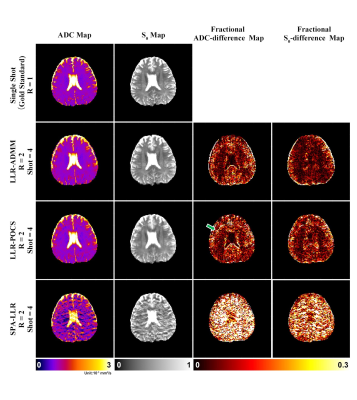
Figure 2 shows the reconstructed DWI image (b-value = 1000s/mm2) for retrospectively undersampled EPIK with different intra-shot undersampling rates. For 2-shot, all three methods achieved similar quality compared with the gold standard. For 4-shot, however, SPA-LLR generated strong artifacts potentially due to a suboptimal SENSE initialization with intra-shot 8-fold undersampling. LLR-ADMM showed reduced noise amplification, improved NRMSE, and reduced aliasing artifacts (green arrows) compared with LLR-POCS.Figure 3 shows reconstruction of the ADC and M0 maps and associated fractional difference maps for the 3 reconstruction methods given retrospective 4-shot acquisition (R=2). The difference between the 3 methods was more apparent in these maps. LLR-ADMM showed the best suppression of image artifacts compared with LLR-POCS and SPA-LLR.
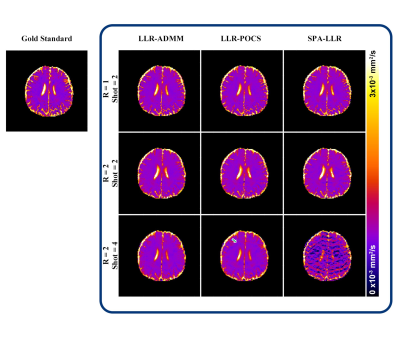
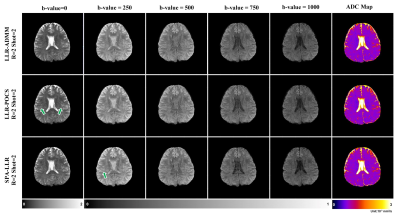
Figure 4 shows reconstruction of the ADC maps in the second healthy subject with prospective undersampling. SPA-LLR showed considerable artifacts with 8-fold intra-shot undersampling. LLR-POCS showed better quality than SPA-LLR, yet there were still some residual aliasing artifacts (green arrows). LLR-ADMM showed the best reconstruction quality. All methods led to increasing loss of fine details with higher undersampling rates.
Figure 5 shows the 1mm isotropic-resolution multi-shot multi-b-value DWI images and the resultant ADC maps reconstructed by the three methods. The intra-shot undersampling rate was 4. LLR-ADMM led to reduced aliasing artifacts compared with LLR-POCS and SPA-LLR.
Discussion and conclusions
We demonstrate a novel acceleration method based on multi-shot EPIK and LLR-ADMM to accelerate multi-b-value DWI. The proposed method showed a reasonable accuracy at intra-shot undersampling rates of 2,4, and 8. By sampling the central k-space in every shot and b-value, the algorithm does not need a “warm-start” based on parallel imaging, which was needed by SPA-LLR and error-prone at higher undersampling rate. Although more experiments are needed to fully validate the method, the preliminary results showed that the method is promising to achieve high-resolution multi-b-value imaging with an efficient acquisition.Acknowledgements
No acknowledgement found.References
1. Rubesova E, Grell A-S, De Maertelaer V, et al. Quantitative diffusion imaging in breast cancer: A clinical prospective study. Journal of Magnetic Resonance Imaging. 2006;24(2):319-324.
2. Schmidt H, Gatidis S, Schwenzer NF, et al. Impact of Measurement Parameters on Apparent Diffusion Coefficient Quantification in Diffusion-Weighted-Magnetic Resonance Imaging. Investigative Radiology. 2015;50(1):46-56.
3. Riexinger A, Martin J, Wetscherek A, et al. An optimized b-value distribution for triexponential intravoxel incoherent motion (IVIM) in the liver. Magnetic Resonance in Medicine. 2021;85(4):2095-2108.
4. Hu Y, Wang X, Tian Q, et al. Multi-shot diffusion-weighted MRI reconstruction with magnitude-based spatial-angular locally low-rank regularization (SPA-LLR). Magn Reson Med. 2020;83(5):1596-1607.
5. Zaitsev M, Zilles K, Shah NJ. Shared k-space echo planar imaging with keyhole. Magnetic Resonance in Medicine. 2001;45(1):109-117.
6. Lima da Cruz G, Bustin A, Jaubert O, et al. Sparsity and locally low rank regularization for MR fingerprinting. Magn Reson Med. 2019;81(6):3530-3543.
7. Zhang T, Pauly JM, Levesque IR. Accelerating parameter mapping with a locally low rank constraint. Magn Reson Med. 2015;73(2):655-661.
8. Lugauer F, Nickel D, Wetzl J, et al. Accelerating multi-echo water-fat MRI with a joint locally low-rank and spatial sparsity-promoting reconstruction. Magnetic Resonance Materials in Physics, Biology and Medicine. 2017;30(2):189-202.
9. Liu Y, Ying L, Chen W, et al. Accelerating the 3D T(1ρ) mapping of cartilage using a signal-compensated robust tensor principal component analysis model. Quant Imaging Med Surg. 2021;11(8):3376-3391.
Figures