4726
Characterizing diffusion properties on extremities of patients with lymphedema by MRI
Yeefan Lee1,2, Kuan-Hung Cho3, Chih-Hsing Tang2, Chia-Wen Chiang3, Shih-Yen Lin4, Chen-Hsiang Kuan5, Chien-Yuan Lin6, Hsiao-Ling Lee6, and Li-Wei Kuo3
1Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 2Department of Medical imaging, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan, 3Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli County, Taiwan, 4Department of Computer Science, National Yang Ming Chiao Tung University, Hsinchu, Taiwan, 5Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan, 6GE Healthcare, Taipei, Taiwan
1Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 2Department of Medical imaging, National Taiwan University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan, 3Institute of Biomedical Engineering and Nanomedicine, National Health Research Institutes, Miaoli County, Taiwan, 4Department of Computer Science, National Yang Ming Chiao Tung University, Hsinchu, Taiwan, 5Department of Surgery, National Taiwan University Hospital, Taipei, Taiwan, 6GE Healthcare, Taipei, Taiwan
Synopsis
Lymphedema is defined as accumulation of fluid and fibroadipose tissues due to disruption of lymphatic flow. Our results demonstrate a significant difference of diffusion measures between lymphedema and normal groups. DKI and DTI derivatives have similar tendency when comparing lymphedema patients with healthy subjects. The diffusion properties may aid the diagnosis of lymphedema and have the potential for evaluating the severity in postoperative follow-up.
Introduction
Lymphedema is a pathologic condition of the lymphatic system in which protein-containing fluid accumulates in the interstitial tissue leading to tissue inflammation, fibrosis, and adipose hypertrophy1. The International Society of Lymphology Lymphedema Staging Guidelines characterizes the severity of lymphedema by two criteria to diagnose and classify lymphedema: the "softness" or "firmness" of the limb (reflecting fibrotic soft tissue changes) and the outcome after elevation2. However, the clinical staging system cannot quantify the severity of lymphedema and delineate the edematous and fibrous tissue distribution within the limbs. Several imaging techniques like lymphoscintigraphy3,4, magnetic resonance lymphangiography (MRL)5, or indocyanine green lymphangiography (ICGL)6 help further assess lymphedema. However, they can only provide qualitative or semi-quantitative results, which highly limits the feasibility of evaluating the preoperative planning and postoperative follow-up. Therefore, how to effectively assess the improvement of lymphatic drainage is of great clinical interest. Diffusion MRI has been widely used in many clinical studies, including breast and body7,8,9,10. It enables to provide quantitative metrics (e.g., mean diffusivity relating to edema and inflammation, and mean kurtosis relating to heterogeneity, cellularity, and inflammation) for clinical diagnosis and disease characterization. This study aims to investigate the role of diffusion imaging for the quantitative assessment of lymphedema.Methods
This study was approved by the local IRB of the National Taiwan University Hospital Hsin-Chu Branch. All volunteers provided informed consent before the commencement of the study. Three normal subjects and one patient with lower extremity lymphedema and two patients with upper extremity lymphedema were enrolled in this study. All patients have clinical stage II to III lymphedema. MRI experiments were performed on a 1.5 T system (SIGNA ARTIST, GE Healthcare, USA) using a 16-channel large flex coil. The diffusion weighted images were acquired using Multishot Diffusion‐Weighted Echo Planar Imaging sequence with Multiplexed Sensitivity Encoding (MUSE) technique11. The b values were set to 0, 200, 400, 800 and 1200 s/mm2 and 15 non-collinear gradient directions were encoded. The image processing procedures include motion correction12 for minimizing the effect from subject movement during MRI scan, followed by ANOLM denoising13. Diffusion tensor imaging (DTI) and Diffusion kurtosis imaging (DKI) were reconstructed based on the algorithms proposed by Basser, P.J., et al.14 and Tabesh, A., et al.15 respectively. Lymphedema is typically confined to the epifascial space of the skin and subcutaneous tissue sparing muscle16,17. Region-of-interests were selected by segmenting the extremity into inner and outer parts (figure 1a and 1e), where inner part mainly contains muscle tissue, and the outer part contains skin and subcutaneous tissue. DTI- and DKI-derived measures, including mean diffusivity (MD), fractional anisotropy (FA) and mean kurtosis (MK), were used for comparison. Student’s t-test was performed. Statistical significance was defined at p<0.05. All values are presented as mean±SD.Results
Figure 1 showed the representative results of DKI-derived MD (figure 1b and 1f), FA (figure 1c and 1g) and MK (figure 1d and 1h) maps from a normal subject and an upper extremity lymphedema patient. Figure 2 shows the comparison between normal subjects and lymphedema patients on DTI- and DKI-derived MD. It can be observed that at outer region both DTI- and DKI-derived MD values from lymphedema patients are significantly higher than that from normal subjects. At inner region, no significant difference between two groups is observed. Figure 3 shows the results of DTI- and DKI-derived FA values. At outer region the FA values from lymphedema patients are significantly lower than that from normal subjects. At inner region there is no significant difference between normal subjects and lymphedema patients. Figure 4 shows the comparison of MK between normal subjects and lymphedema patients. The MK value from lymphedema patients is obviously lower than that from normal subjects at outer region but is similar to that from normal subjects at inner region.Discussion & conclusion
Our preliminary results demonstrate that higher MD values, lower FA and MK values present at the outer part of the extremities of the lymphedema patients than healthy controls. These results demonstrate the feasibility for the diagnosis of extremity lymphedema using diffusion measures. The inner part of the lymphedema extremities shows similar diffusion properties to healthy controls. This result indicates that lymphedema does not occur in muscle tissue, consistent with the prior clinical observations16,17. Our results also show that DTI-derived MD and FA values are slightly different from DKI derivatives owing to different modeling of diffusion signals. However, we can still conduct the same conclusion since the tendency is similar when comparing lymphedema patients with healthy subjects. In conclusion, derivatives from DTI and DKI can be applied to differentiate lymphedema patients from healthy controls. The diffusion properties may be helpful in the diagnosis of lymphedema and have the potential for evaluating the severity in postoperative follow-up.Acknowledgements
I would like to thank all those who are related to this project.References
- Grada AA, Philips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol 2017;77:1009-20.
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53(1):3-19.
- Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003 Jan;44(1):43-57.
- Maclellan RA, Zurakowski D, Voss S, Greene AK. Correlation Between Lymphedema Disease Severity and Lymphoscintigraphic Findings: A Clinical-Radiologic Study. J Am Coll Surg. 2017 Sep;225(3):366-370.
- Werner GT, Scheck R, Kaiserling E. Magnetic resonance imaging of peripheral lymphedema. Lymphology. 1998 Mar;31(1):34-6. PMID: 9561511.
- Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine Green Lymphography Findings in Limb Lymphedema. J Reconstr Microsurg. 2016 Jan;32(1):72-9.
- Chhetri A., et al., Current and Emerging Magnetic Resonance-Based Techniques for Breast Cancer. Front Med (Lausanne). 2020 May 12;7:175.
- Tang L., et al., Diffusion MRI of cancer: From low to high b-values. J Magn Reson Imaging. 2019 Jan;49(1):23-40.
- Liu W., et al., Histogram analysis of diffusion kurtosis imaging in the differentiation of malignant from benign breast lesions. Eur J Radiol. 2019 Aug;117:156-163.
- Rosenkrantz AB, et al., Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging. 2015 Nov;42(5):1190-202.
- Chen, N.-K., et al., A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). NeuroImage, 2013. 72: p. 41-47.
- Oakes, T.R., et al., Comparison of fMRI motion correction software tools. Neuroimage, 2005. 28(3): p. 529-43.
- Manjón, J.V., et al., Adaptive non-local means denoising of MR images with spatially varying noise levels. J Magn Reson Imaging, 2010. 31(1): p. 192-203.
- Basser, P.J., J. Mattiello, and D. LeBihan, Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B, 1994. 103(3): p. 247-54.
- Tabesh, A., et al., Estimation of tensors and tensor-derived measures in diffusional kurtosis imaging. Magn Reson Med, 2011. 65(3): p. 823-36.
- Haaverstad R. Nilsen G. Rinck PA. Myhre HO. The use of MRI in the diagnosis of chronic lymphedema of the lower extremity. Int Angiol 1994: 13(2): 115-18.
- Case TC, Witte CL. Witte MH, Unger EC. Williams WH, Magnetic resonance imaging in human lymphedema: comparison with lymphangioscinngraphy. Magn Reson Imaging 1992: 10(4): 549-58.
Figures
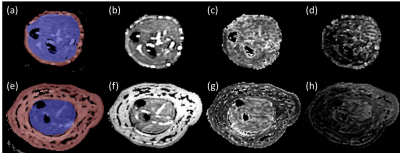
Figure 1. Representative DKI-derived maps from a
healthy subject (upper row) and an upper extremity lymphedema patient (lower
row). The inner (blue region) and outer (red region) ROIs are shown in a and e.
b and f are MD maps; c and g are FA maps; d and h are MK maps.
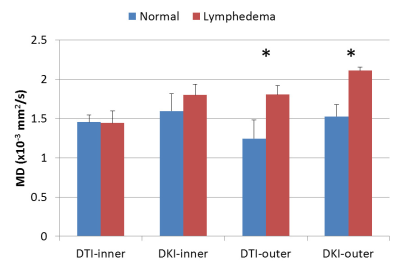
Figure 2. The comparison of DTI- and DKI-derived
MD values measured from normal and patient groups. * represents p<0.05.
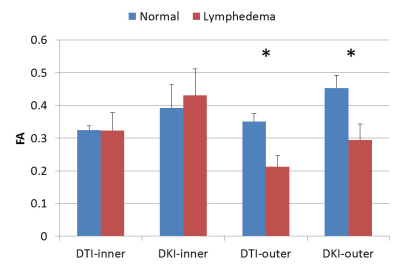
Figure
3. The comparison of DTI- and DKI-derived FA values measured from normal and
patient groups. * represents p<0.05.
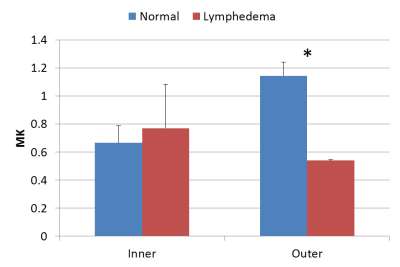
Figure
4. The comparison of DKI-derived MK values measured from normal and patient
groups. * represents p<0.05.
DOI: https://doi.org/10.58530/2022/4726