4713
The Effect of Sliding Window Reconstruction with Multiplexed-Sensitivity Encoding on Multi-shot Resting-State fMRI Data1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2GE Healthcare, Taiwan, Taiwan, 3Department of Biomedical Engineering, The Chinese University of Hong Kong, Hong Kong, Hong Kong
Synopsis
High-resolution 4-shot multi-echo resting-state fMRI (RS-fMRI) can be achieved by acquiring data in a sliding window manner, and thus the composite full k-space for MUSE reconstruction can be obtained by combining the segments from the consecutive time points. However, the sliding window operation may smooth the time courses and lead to a similar effect as applying a moving average filter. In this study, we aimed to investigate the effect of k-space data sharing from consecutive time points acquired with different TRs, and to determine a feasible TR for the acquisition and reconstruction scheme for high-resolution 4-shot multi-echo RS-fMRI with MUSE.
Introduction
In our previous study, we proposed a multi-shot acquisition scheme with sliding window to enable MUSE reconstruction1 for high-resolution multi-echo resting-state fMRI (RS-fMRI), by pre-combining the k-space segments acquired from consecutive time points2 into a full k-space data. Compared to the conventional single-shot RS-fMRI data, time series derived from composite full k-space data may reveal smoothing effect on time courses that may be similar to applying moving average filter (MAF)3 to the signal. The application of MAF on time signal may cause power decrease in frequency domain. In addition, the cut-off frequency depends on TR and window size (equal to the number of shots used in multi-shot fMRI with MUSE2). With a desired number of shots (i.e., 4), TR is the dominant factor that theoretically determines the power decrease effect in frequency spectrum. Since the bandwidth for RS-fMRI analysis was reported within the range of 0.01-0.1Hz4-8, any influence on frequency spectrum by incorporating sliding window (i.e., data sharing) into RS-fMRI data reconstruction needs to be considered. In this study, we aimed to investigate the effect of sliding window on RS-fMRI data reconstruction and to determine the feasible TR for 4-shot RS-fMRI data using MUSE1, 2.Methods
TheoryThe operation of MAF is described by the following equation3:
$$y[n] =\sum_\left(m=0\right)^\left(M-1\right)x[n+m]$$
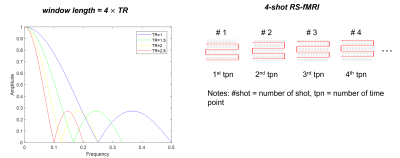
Where each point of the output signal y[ ] is produced by averaging a given number M of consecutive points from the input signal x[ ]. The typical TR used for RS-fMRI acquisition is 2s9 and shorter TR (e.g., around 1.1s) can be achieved using multiband EPI10. The corresponding window length of MAF is calculated as #shot TR for sliding window reconstruction. With a given number of shots equal to 4, the first cut-off frequency is decreased when there is an increase in TR (Figure 1). The roll-off of the frequency response within the bandwidth of 0.01-0.1Hz is sharper with the increase of TR, demonstrating that a lower percentage of spectrum power loss is associated with a shorter TR.
Data acquisition and simulation
Two sets of RS-fMRI data were acquired from 4 healthy subjects at two different TRs with a single-shot EPI (ssEPI) sequence on a 3T GE MRI scanner, using a 48-channel head coil. The parameters were as follows: TR = 1000/2000ms, TE = 30ms, matrix size = 64$$$\times$$$64, number of slices = 24, FOV = 24.3cm, slice thickness = 3.8mm, isotropic voxel size = 3.8mm3, multiband factor = 2. T1-weighted images were acquired using a 3D-FSPGR sequence for anatomical registration. Two sets of GRE images were also acquired from each individual subject to generate two sets of coil sensitivity profiles with different random noise distribution for the subsequent simulation. The original RS-fMRI signals using ssEPI were used as the gold standard for all comparisons conducted in this study.
Simulation 1: To estimate the effect of MAF on time courses, the original images acquired at TR = 1s/2s were temporally smoothed by a 4-point MAF to respectively generate four datasets for the following analysis and group comparison.
Simulation 2: To estimate the effect of composite full k-space from consecutive time points using highly-accelerated data, the original images were modulated by the first set of coil sensitivity profiles and 2D phase error maps, and then transformed to k-space for generating multi-coil k-space data. Afterward, the 4-fold subsampled data were generated with the sliding window acquisition according to the acquisition strategy proposed in our previous study2. The simulated data were then reconstructed by MUSE with the second set of coil sensitivity profiles.
Data evaluation
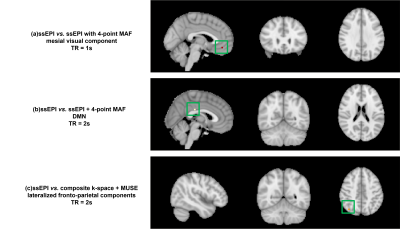
The RS-MRI data were analysed using MELODIC implemented in FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl). After the standard pre-processing steps, the group ICA with dual regression were performed among the following three group pairs (Figure 2) to compare the group differences for the data acquired with either TR = 1s or 2s, respectively.
Results
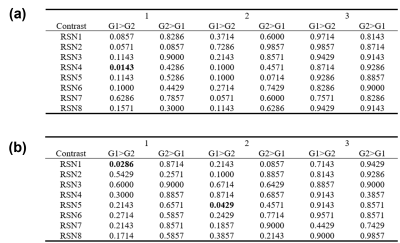
Without considering Bonferroni correction, the p-values in Figure 3 indicated that the significant increases can be observed in mesial visual component (p<0.0143) in the original ssEPI data acquired with TR = 1 in 1st group pair. With TR = 2s, the significant differences were found in DMN (p<0.0286) in 1st group pair and lateralized fronto-parietal component (p<0.0429) in 2nd group pair (Figure 4).Discussion and conclusion
With consideration of Bonferroni correction, the p-value that indicates the significant differences should be 0.5/(8x2) = 0.03125 because only eight resting state networks11 were observed in this study. In this case, the significant differences were only shown in 1st group comparison (ssEPI vs. ssEPI + 4-point MAF). In 2nd group comparison (ssEPI vs. composite k-space + MUSE), no significant differences were found with Bonferroni correction. Notably, no significant differences were observed in 2nd group comparison with TR = 1s whether Bonferroni correction was considered or not, demonstrating that shorter TR had less effect on frequency spectrum. Furthermore, no significant differences were shown in 3rd group comparison (composite k-space + MUSE vs. ssEPI +4-point MAF), suggesting that the two processing methods had similar effect on the RS-fMRI signal. To conclude, the combination of k-space data sharing from consecutive time points with subsequent MUSE reconstruction is feasible for 4-shot RS-fMRI when TR is equal to 2s or shorter.Acknowledgements
The work was in part supported by grants from Hong Kong Research Grant Council (GRFs 17121517, 17106820, and 17125321).References
1.Chen, N.-k., A. Guidon, H.-C. Chang, and A.W. Song. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimage. 2013; 72: 41-47.
2.CHEN, S., M. Chu, Q. Chan, et al. Multi-Echo Multi-Segment EPI Based fMRI Using Sliding-Window Acquisition and Multiplexed Sensitivity Encoding (MUSE). in International Society for Magnetic Resonance in Medicine, 28th Annual Meeting. 2020.
3.Smith, S.W. The scientist and engineer's guide to digital signal processing. 1997.
4.Biswal, B., F. Zerrin Yetkin, V.M. Haughton, and J.S. Hyde. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magnetic resonance in medicine. 1995; 34(4): 537-541.
5.Biswal, B.B., J.V. Kylen, and J.S. Hyde. Simultaneous assessment of flow and BOLD signals in resting‐state functional connectivity maps. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo. 1997; 10(4‐5): 165-170.
6.Cordes, D., V.M. Haughton, K. Arfanakis, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American journal of neuroradiology. 2001; 22(7): 1326-1333.
7.Lowe, M.J., M. Dzemidzic, J.T. Lurito, V.P. Mathews, and M.D. Phillips. Correlations in low-frequency BOLD fluctuations reflect cortico-cortical connections. Neuroimage. 2000; 12(5): 582-587.
8.Lowe, M., B. Mock, and J. Sorenson. Resting state fMRI signal correlations in multi-slice EPI. Neuroimage. 1996; 3(3): S257.
9.Yuan, B.-K., J. Wang, Y.-F. Zang, and D.-Q. Liu. Amplitude differences in high-frequency fMRI signals between eyes open and eyes closed resting states. Frontiers in human neuroscience. 2014; 8: 503.
10.Podgórski, P., M. Waliszewska-Prosół, A. Zimny, M. Sąsiadek, and J. Bladowska. Resting-State Functional Connectivity of the Ageing Female Brain—Differences Between Young and Elderly Female Adults on Multislice Short TR rs-fMRI. Frontiers in neurology. 2021; 12.
11.Rosazza, C. and L. Minati. Resting-state brain networks: literature review and clinical applications. Neurological sciences. 2011; 32(5): 773-785.
Figures