4679
A preliminary study of real-time imaging for MR guided vascular intervention and image quality assessment1MR, Taiyuan Central Hospital of Shanxi Medical University, Shanxi, China, 2Physiology, Shanxi Medical University, Shanxi, China, 3Medical Imaging, Shanxi Medical University, Shanxi, China, 4MR Scientific Marketing, Siemens Healthineers, Beijing, China, 5MR Customer Application Specialist, Siemens Healthineers Digital Technology (Shanghai) Co. Ltd., Beijing, China
Synopsis
This study conducted a preliminary phantom study of MRI-guided vascular intervention, and evaluated the image quality of MR real-time sequences for MR compatible guidewire and balloon imaging with a 3D printed aorta phantom in a 3T MRI scanner. The results showed that the two real-time imaging sequences have sufficient guiding ability to guide the guidewire and balloon to the expected position. After comprehensive evaluation, it was concluded that the FLASH sequence was better than the True FISP sequence.
Background
Interventional MRI have significant benefits for image-guided intravascular intervention and related vascular procedures1-3. Real-time MRI could provide a radiation-free alternative to X-ray guidance of cardiovascular catheterization, enables superb tissue contrast depiction in multi-contrast imaging without administration of contrast agent. In this study, we evaluated the image quality of two MR real-time sequences, fast low angle shot (FLASH) and true fast imaging with steady-state precession (True FISP), for MR guided vascular intervention using MR compatible guidewire and balloon in a 3D printed aorta phantom4-6.Methods
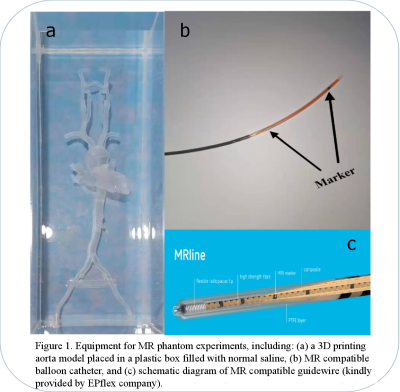
In this study, an MR guided intervention system were setup, which consisted of a 3T scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) and MRI compatible monitor (Fuqing Medical Technology Co., Ltd, Hefei, Anhui, China). All experiments were performed using a peripheral balloon catheter (PTA35-6040B, DK MEDTECH, Suzhou, Jiangsu, China), which filled with 0.8ml 1% Gadodiamide mixed with normal saline and magnetic resonance compatible guidewire (diameter=0.035 in, length=150cm) (EPflex, Dettingen an der Erms, Germany). Guidewire consisted of a high-strength para-aramid synthetic fiber core, surrounded by a bending-resistant high-performance polymer and a hydrophilic coating, the markers in the guidewire were discretely mosaicked for navigation. All experiments were performed in a 3D printed aorta phantom printed by 3D printer (SPS250j, Shaanxi Hengtong Intelligent Machine Co., Ltd, Shaanxi, China). The aorta phantom was made of transparent resin with CTA data from a normal adult (Figure 1). The interventional procedure was guided and recorded by FLASH and True FISP. The parameters of the real-time imaging sequence were: (1) FLASH, TR/TE =6.8/2.65ms, FA=12º, FOV=360×147mm, Slice thickness=10mm, Phase partial Fourier=6/8, bandwidth=230Hz/Px, (2) True FISP, TR/TE =321.35/1.88ms, FA=49º FOV=360×144mm, Slice thickness=10mm, bandwidth=1116Hz/Px7,8.The real-time images were evaluated objectively and subjectively. For objective assessment, the image signal-to-noise ratio (SNR), contrast noise ratio (CNR), geometric distortion, and image uniformity of two sequences were measured. The signal intensity and noise at different positions of the image were measured and their mean value was taken to calculate the SNR. The SNR was calculated by: $$SNR=Signal nomal saline/SD noise$$
The CNR between gadolinium-filled balloon and normal saline in the phantom was measured. The CNR was calculated by:$$CNR=|Signal balloon - Signal nomal saline|/SD noise$$
Regarding image uniformity, most areas of multiple images were measured, and the mean value of their uniformity was calculated. The image uniformity was calculated by: $$U={1-(Signal max-Signal min)/(Signal max+Signal min)}×100%$$
In terms of geometric distortion, the actual length of the gadolinium balloon and its length in the image was measured, and then the geometric distortion was calculated by: $$GD=|Length image-Length actual|/Length image×100%$$
For subjective assessment, Likert scoring was applied to assess the image sharpness, image distortion, and artifacts of all sequences. The images quality were scored subjectively by two experienced radiologists with more than six-year practice. The evaluation criteria were as follows: 1 points = poor image sharpness with serious distortion or artifacts, 2 points = medium image sharpness with medium distortion or artifact, 3 points = good image sharpness with slight distortion or artifacts, 4 points = excellent image sharpness with no distortion or artifacts9.
The results of the objectively and subjectively analyses were compared between the two sequences using independent sample T-test by IBM SPSS Statistics 26.
Results
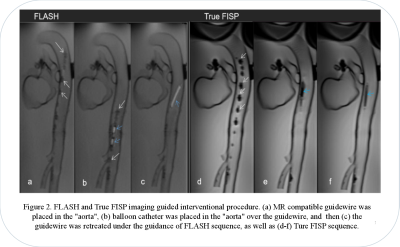
Both sequences temporal resolution was 2 frames/s and spatial resolution was 1.1×1.1×10mm (Figure 2). For objective assessment (Table 1), the two sequences (FLASH vs True FISP) showed significant different SNR (102±16 vs 618±137, p<0.05) and CNR (73.5±18 vs 162.2±46, p<0.05). Image uniformity (80%±6% vs 74%±4%, p=0.133) and geometric distortion (2%±1%, 2%±1%, p=0.717) did not showed significant difference. Two radiologists reached agreement on subjective scoring. The subjective scores of two sequences did showed significant difference, and the FLASH score was higher than the True FISP score (FALSH=3.7±0.5, True FISP=3.1±0.9, p<0.05).The artifacts of the guidewire were moderate for FLASH, and the balloon was high signal. In True FISP, the artifacts of the guidewire were severe, the balloon was low signal and blurred by the artifacts of the guidewire.
Table 1 Objective evaluation results
| | SNR | CNR | Image uniformity | geometric distortion |
| FLASH | 102±16 | 73.5 ±18 | 80% ±6% | 2% ±1% |
| True FISP | 618 ±137 | 162.2 ±46 | 74% ±4% | 2% ±1% |
Discussion
Both sequences have sufficient SNR, CNR, image uniformity and the degree of geometric distortion was also within the acceptable range. The FLASH sequence has sufficient guiding ability in the actual application process, the artifacts of the guidewire were moderate. Although the SNR and CNR were higher for True FISP, the artifacts of the guidewire were severe, and blurred display of balloon.Conclusions
In conclusion, the study demonstrates the MR real-time imaging, FLAH sequence is feasible of guiding cardiovascular interventions.Acknowledgements
No acknowledgement found.References
1. Blanco RT, Ojala R, Kariniemi J, et al. Interventional and intraoperative MRI at low field scanner--a review. Eur J Radiol. 2005;56(2):130-42.
2. Rogers T, Lederman RJ. Interventional CMR: Clinical applications and future directions. Curr Cardiol Rep. 2015;17(5):31.
3. Kim HG, Choi JW, Yoon SH, et al. Image quality assessment of silent T2 PROPELLER sequence for brain imaging in infants. Br J Radiol. 2018;91(1083):20170680.
4. Velasco Forte MN, Roujol S, Ruijsink B, et al. MRI for Guided Right and Left Heart Cardiac Catheterization: A Prospective Study in Congenital Heart Disease. J Magn Reson Imaging. 2021;53(5):1446-1457.
5. Tzifa A, Krombach GA, Kramer N, et al. Magnetic resonance‐guided cardiac interventions using magnetic resonance‐compatible devices: A preclinical study and first in man congenital interventions. Circ Cardiovasc Interv. 2010;3(6):585‐592.
6. Campbell-Washburn AE, Rogers T, Stine AM, et al. Right heart catheterization using metallic guidewires and low SAR cardiovascular magnetic resonance fluoroscopy at 1.5 Tesla: first in human experience. J Cardiovasc Magn Reson. 2018;20(1):41.
7. Ratnayaka K, Faranesh AZ, Hansen MS, et al. Real-time MRI-guided right heart catheterization in adults using passive catheters. Eur Heart J. 2013;34(5):380-9.
8. Eggebrecht H, Kühl H, Kaiser GM, et al. Feasibility of real-time magnetic resonance-guided stent-graft placement in a swine model of descending aortic dissection. Eur Heart J. 2006;27(5):613-20.
9. Kahlert P, Parohl N, Albert J, et al. Towards real-time cardiovascular magnetic resonance guided transarterial CoreValve implantation: in vivo evaluation in swine. J Cardiovasc Magn Reson. 2012;14(1):21.
Figures