4676
Longitudinal dynamic changes in DTI metrics after repetitive mild traumatic brain injury with different intervals1Department of Biomedical Imaging and Radiological Sciences, National Yang-Ming Chiao-Tung University, Taipei, Taiwan, 2Department of Medical Imaging and Radiological Sciences, College of Medicine, Chang-Gung University, Taoyuan, Taiwan
Synopsis
Long-term effects of repetitive mild traumatic brain injury (rmTBI) with the short versus long interinjury interval were explored using quantitative DTI and immunohistological staining. Significant changes in DTI metrics under the impact site and the lateral part of white matter in the ipsilesional brain after rmTBI may suggest the exacerbated microstructural damage induced by repetitive injury with the short interval.
Introduction
Repetitive mild traumatic brain injury (rmTBI) is a growing public health concern, especially among athletes and military personnel.1 Repetitive mTBI may result in cumulative and persisting neurological deficits, and subsequent neurodegenerative diseases.2 Most of studies assumed that outcomes of rmTBI may be dependent on the time interval of repetitive injury.3 However, the long-term effect on microstructural changes after rmTBI with different interinjury intervals was yet well documented. In this study, we used the closed-head injury (CHI) rat model imitating uncomplicated mTBI in clinical to replicate rmTBI with different interinjury intervals.4 To address the effect of interinjury interval following rmTBI, longitudinal DTI MRI and neuropathological examination were performed and analyzed. Our findings showed that changes in DTI metrics under the impact region and the white matter are dependent on the interinjury interval, implying that even without significant brain lesion, the microstructural injury is exacerbated following rmTBI administered within a relatively short time.Methods
Male Sprague–Dawley rats were anesthetized with Chloral Hydrate for sham surgery (sham group, n = 5) and rCHI for 4 hits injury with the interval of 6 h (short interval group, n = 4) or 24 h (long interval group, n = 5) targeting on the skull on top of the left motorsensory cortex (1.5 mm posterior and 2.5 mm lateral to bregma). A weight of 600 g was dropped from a height of 1 m through a stainless-steel tube to the secured impactor aiming to the metal helmet cemented on the skull.4 Longitudinal MRI was performed at day 50 and 90 after rCHI. Animals were anesthetized under ~1.2% isoflurane for acquisition on a Bruker 7T PharmaScan scanner. T2-weighted images using a rapid acquisition with a relaxation enhancement sequence (TR/TE = 3600/40 ms, FOV = 2.0 × 2.0 cm, matrix size = 256 × 256, 16 slices, slice thickness = 1 mm) were obtained to acquire anatomic images for rodent models. Diffusion tensor images were acquired with the same geometry using the 4-shot spin-echo EPI with TR/TE = 3000/28 ms, matrix size = 96 × 96, δ/△ = 5/15 ms, number of B0 = 5, number of directions = 30, b-value = 1000 s/mm2, number of averages = 4. Immunohistochemical staining used anti-glial fibrillary acidic protein (GFAP), hexaribonucleotide binding protein-3 (NeuN); myelin basic protein (MBP) for astrocytes, neurons and myelinated fibers, respectively, were examined in subgroup of animals after the last MRI acquisition. Paired sample t-test was employed to determine the differences in ROIs between two hemispheres (P < 0.05). Two-way ANOVA was used to determine the differences among groups of animals and among different time points (P < 0.05).Results & Discussion
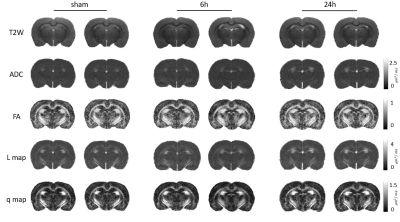
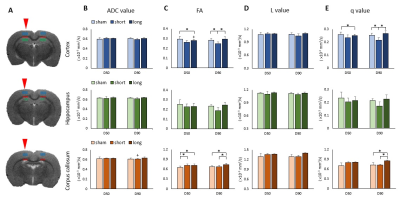
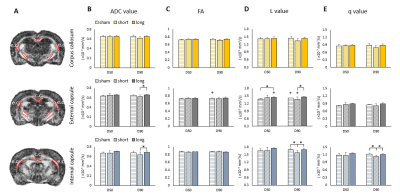
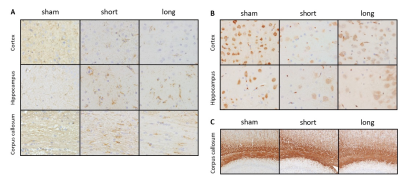
There was no focal brain contusions or hemorrhage at 50 or 90 days after rCHI (Fig. 1). No significant difference in ADC and L value was observed in any ROI under the impact site (Fig. 2B and D). In the ipsilateral cortex, significant lower FA and q value compared with the sham were shown at day 90 after rCHI with short interval and at day 50 after rCHI with long interval (Fig. 2C and E). In the ipsilateral corpus callosum (c.c.), a significant higher FA value compared with the sham was shown at day 50 after rCHI with both intervals and lasted at day 90 after rCHI with long interval, which was also higher than that in the short interval group (Fig. 2C). In addition, a significant higher q value was observed at day 90 after rCHI with long interval compared with that in the sham and short interval group (Fig. 2E). No significant difference was found in the hippocampus. The results suggested that significant changes in DTI metrics were still descent under the impact site at the chronic phase following rmTBI regardless to the interinjury interval. No significant difference in DTI metrics was observed in the medial part of c.c. (Fig. 3B-E). In the ipsilateral external capsule (e.c.), significant lower ADC value and L value compared with long interval group were shown at day 90 after rCHI with short interval (Fig. 3B and D). A significant higher L value at day 50 after rCHI with long interval was observed compared with the sham (Fig. 3D). In the ipsilateral internal capsule (i.c.), significant lower ADC, L and q value compared with long interval group were observed at day 90 after rCHI with short interval (Fig. 3B, D and E). Moreover, significant lower L and q value compared with the sham were found at day 90 after rCHI with short interval, suggesting the disruption of white matter (Fig. 3D and E).5 Our results demonstrated that changes in diffusion characteristics in the white matter were majorly found in the lateral part of the ipsilateral hemisphere and more prominent after rCHI with short interval. Increase of infiltrating astrocytes (Fig. 4A) and loss of neurons (Fig. 4B) in the gray matter were found under the impact site after rCHI with short interval, suggesting that neuroinflammation was exacerbated after injury with short interval.6 Our future study aims to explore how the impact affects the contralateral hemisphere and correlates with behavioral outcomes after rCHI with different intervals.Acknowledgements
This study was funded in part by Ministry of Science and Technology (MOST 109-2314-B-A49A-550 and MOST 109-2314-B-010-067-MY3), Taipei, Taiwan.References
1. Aungst SL, Kabadi SV, Thompson SM, Stoica BA, Faden AI. Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 2014; 34(7): 1223-32.
2. Wortman RC, Meconi A, Neale KJ, et al. Diffusion MRI abnormalities in adolescent rats given repeated mild traumatic brain injury. Ann Clin Transl Neurol 2018; 5(12): 1588-98.
3. Donovan V, Kim C, Anugerah AK, et al. Repeated mild traumatic brain injury results in long-term white-matter disruption. J Cereb Blood Flow Metab 2014; 34(4): 715-23.
4. Kao YJ, Lui YW, Lu CF, Chen HL, Hsieh BY, Chen CY. Behavioral and Structural Effects of Single and Repeat Closed-Head Injury. AJNR Am J Neuroradiol 2019; 40(4): 601-8.
5. Price SJ, Pena A, Burnet NG, et al. Tissue signature characterisation of diffusion tensor abnormalities in cerebral gliomas. Eur Radiol 2004; 14(10): 1909-17.
6. Bolton AN, Saatman KE. Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J Neuropathol Exp Neurol 2014; 73(10): 933-47.
Figures