4675
Evidence for nigral neuromelanin abnormalities in rat brain in the methamphetamine addiction assessed by neuromelanin-sensitive MRI1The Second Xiangya Hospital of Central South University, Changsha, China, 2MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
NM-MRI is a newly developed magnetic resonance imaging technique, which indirectly measures dopamine synthesis and demonstrate neuromelanin-related contrast. In this study, ten rats with acute methamphetamine exposure underwent a NM-MRI scan at different time points. The NM-MRI data of four brain regions were measured and compared. The results showed significantly higher NM-MRI signal in substantia nigra compared to other brain regions, and the NM-MRI signal gradually increased over time in almost all measured brain regions. These findings demonstrated the potential of NM-MRI as biomarker and the predictive value of NM-MRI for dopamine function.
Abstract
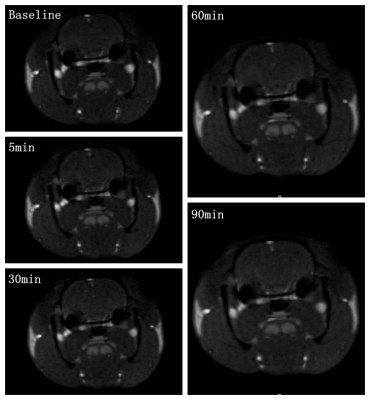
Objective Neuromelanin-sensitive MRI (NM-MRI) purports to detect the content of neuromelanin (NM), a product of dopamine metabolism that accumulates in the substantia nigra (SN). Prior work has shown that NM-MRI provides a marker of SN integrity in neurodegeneration[1, 2]. NM-MRI signal is reliably decreased in the SN of patients with neurodegeneration of Parkinson’s disease (PD)[3]. Moreover, some initial studies have shown that cocaine use disorders lead to increased neuromelanin signal [4]. In this study, we aimed to investigate the ability of NM-MRI to measure neuromelanin concentration in the patients with methamphetamine addiction, a non-neurodegenerative disorder involving dopamine dysfunction, to explore whether NM-MRI signals can be indirectly used as potential biomarker of dopamine function in non-neurodegenerative diseases.Methods A total of ten rats were included in this experiment. To prevent motion artifacts during performing MRI examinations, all rats were anesthetized with a dose of 2ml/kg (0.3g/ml pentobarbital sodium solution) before each scan. Scans were performed on a 3.0 T MRI system (uMR 790, United Imaging Healthcare, Shanghai, China) with a 12-channel rat coil. The study protocol included the following sequences: (1) 3D T1-weighted gradient-recalled echo sequence (repetition time (TR)/echo time (TE) = 10.16/4.6 msec, flip angle (FA) = 12°, 26 slices, slice thickness = 1.2 mm, field of view (FOV) = 50 × 72 mm, matrix = 167 × 240); (2) T2-weigthed fast spin echo (FSE) sequence (TR/TE = 6372/103.36 msec, FA = 145°, 26 slices, slice thickness = 1.2 mm, FOV = 50 × 72 mm, matrix = 250 × 360); (3) NM-MRI sequence. NM-MRI was performed by using the GRE sequence with magnetization transfer (MT) pulse: TR/TE = 62/5.2 msec, FA = 40°, 6 slices, slice thickness = 1.2 mm, FOV = 50 × 72 mm, matrix = 167 × 240, magnetization transfer frequency offset = 2000 Hz and duration = 10 msec. Baseline NM-MRI and T1-weighted images were acquired from all the rats without acute methamphetamine exposure. One acute intra-jugular injection of methamphetamine (0.2 mg/kg of body weight) were given after baseline NM-MRI scanning. The rats were performed NM-MRI scan at 5, 30, 60 and 90 minutes after injection (Figure 1). Regions of interest (ROIs), including the caudate putamen (CPU), nucleus accumbens (Nac), hippocampus, and SN were manually drawn by one experienced radiologist. Among these ROIs, the mean NM-MRI signals at different time points were recorded. Statistical analysis was performed using IBM SPSS 22. For statistical analysis, the NM-MRI signals of four brain regions (CPU, Nac, hippocampus, and SN) at different time points of five groups (baseline, 5min, 30min, 60min, 90min) were compared using ANOVA. Significance was at the 95% confidence level.
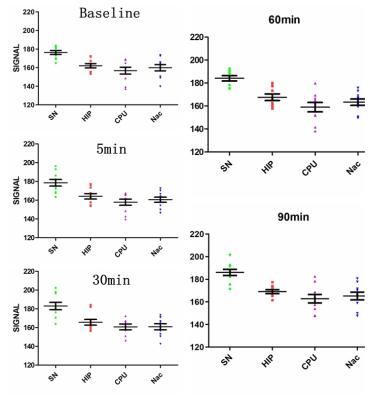
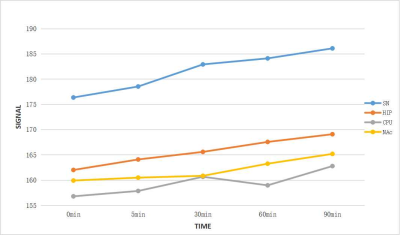
Results At all the time points (baseline, 5min, 30min, 60min, 90min), NM-MRI signal of SN was significantly higher than that of other brain regions (P<0.05) (Figure 2). It suggested that neuromelanin was mainly deposited in the substantia nigra, which was consistent with previous research[3, 5]. Moreover, Acute methamphetamine exposure resulted in elevated levels of NM-MRI signal in all the brain regions over time, although the magnitude of increase varied between brain regions (Figure 3). For one thing, compared with Nac, NM-MRI signal of SN increased at 30 min with statistical significance (P<0.05). For another, Compared with CPU, NM-MRI signal of SN increased at 60 minutes with statistical significance (P<0.05).
Discussion &Conclusion Our results indirectly indicated that increased dopamine can lead to increased accumulation of neuromelanin in multiple brain regions, which can be revealed by NM-MRI. Given that previous imaging studies show decreased dopamine signaling in the SN of patients with neurodegeneration of PD[3], the finding of increased NM-MRI signal in the substantia nigra provides additional insight into the pathophysiology of methamphetamine exposure. One interpretation is that methamphetamine exposure is associated with a redistribution of dopamine between cytosolic and vesicular pools, leading to increased accumulation of neuromelanin[4]. Another explanation is that NM accumulate and increase with age, and melanin synthesis itself acts as a protective mechanism, clearing out excess cytoplasmic catecholamines and increasing melanin synthesis[5]. In conclusion, this study suggested that NM-MRI signals can indirectly reflect the concentration of neuromelanin in brain tissues. NM-MRI can serve as a powerful imaging tool for interrogating the dopamine system in addiction, and is a practical tool that could have diverse research and clinical applications to detect pathological changes in methamphetamine addiction and related disorders.
Acknowledgements
No AcknowledgementsReferences
References
1. Reimão, S., et al., Substantia nigra neuromelanin magnetic resonance imaging in de novo Parkinson's disease patients. Eur J Neurol, 2015. 22(3): p. 540-6.
2. van der Pluijm, M., et al., Reliability and Reproducibility of Neuromelanin-Sensitive Imaging of the Substantia Nigra: A Comparison of Three Different Sequences. J Magn Reson Imaging, 2021. 53(3): p. 712-721.
3.Cassidy, C.M., et al., Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci U S A, 2019. 116(11): p. 5108-5117.
4. Cassidy, C.M., et al., Evidence for Dopamine Abnormalities in the Substantia Nigra in Cocaine Addiction Revealed by Neuromelanin-Sensitive MRI. Am J Psychiatry, 2020. 177(11): p. 1038-1047.
5.Zecca, L., et al., The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson's disease. FEBS Lett, 2002. 510(3): p. 216-20.
Figures