4674
Optogenetic fMRI investigation of the anterior cingulate cortex in pain processing
Taeyi You1,2, Jeong-Yun Lee2, Choong-Hee Lee2, Geun Ho Im2, Heewon Seo2, Choong-Wan Woo1,2,3, and Seong-Gi Kim1,2,3
1Biomedical Engineering, Sungkyungkwan University, Suwon, Korea, Republic of, 2Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 3Intelligent Precision Healthcare Convergence, Sungkyungkwan University, Suwon, Korea, Republic of
1Biomedical Engineering, Sungkyungkwan University, Suwon, Korea, Republic of, 2Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 3Intelligent Precision Healthcare Convergence, Sungkyungkwan University, Suwon, Korea, Republic of
Synopsis
Pain involves a multidimension network of brain circuits related to both somatosensation and cognitive-motivational dimension. Mouse fMRI allows for the in-vivo brain-wide functional mapping that can help explore the pain circuits at a systems level. We utilized transgenic mice in which we suppressed the anterior cingulate cortex (ACC) with optogenetics to better understand its role in pain. Our behavior and fMRI results show that the ACC is involved in the cognitive-motivational dimension of pain, but not in the sensation of pain. In addition, we detected other brain regions as potential targets related to pain hypersensitivity with fMRI.
Introduction
Pain is a sensory and affective experience signaling actual or potential tissue damage for survival in threatening situations. Traditionally, pain processing in the brain is known to have a sensory-discriminative dimension, such as sensory information of location and quality, and an affective-motivational dimension, which establishes the unpleasantness of pain and aversive experiences to avoid the threat of pain. This makes exploring pain circuits at systems-level challenging with traditional neuroscience tools as they involve multiple brain regions1. Mouse fMRI allows for in-vivo brain-wide functional mapping and combining it with optogenetics allows us to investigate downstream circuitry of a targeted nuclei. Here, we focused on the anterior cingulate cortex (ACC) as it is a known critical hub for pain-related multidimensional experience. We utilized transgenic channelrhodopsin2 (ChR2) mice to suppress the ACC to better understand its role in hypersensitivity to pain and nociception.Methods
ACC optic fiber implantation (105 µm inner core diameter) was performed on in-house bred transgenic mice expressing ChR2 in GABAergic interneurons (VGAT-ChR2) under ketamine/xylazine with the following coordinates: 1.0 mm A/P, 0.3 mm M/L, 1.0 mm D/V relative to bregma. Mice were allowed to recover to 4 weeks before behavior experiments and fMRI. All optogenetic stimulation during behavior and fMRI was applied with a 473 nm laser at 20Hz, 20% duty cycle with 3mW intensity at the fiber tip. Activation of inhibitory neurons induces inhibition of excitatory neurons in ACC and downstream networks. To assess thermal-evoked pain, paw withdrawal latency was measured using the Hargreaves method in which the mouse's hindpaw was heated via infrared stimulation. Baseline paw withdrawal latency was measured with or without ACC inhibition during stimulation. Next, pain models were induced by either injecting capsaicin, for acute, or complete Freund’s adjuvant (CFA), for chronic pain, into the plantar surface of the right hindpaw. The number of times the mice licked their paw was counted, and the thermal pain threshold was also measured with and without ACC inhibition as scheduled in Fig. 1A. Optogenetic fMRI experiments were conducted at 15.2T Bruker Biospec System under dexmedetomidine/isoflurane anesthesia as previously described2. Chronic pain and naive models were scanned with GE-EPI parameters of[KSG1] TR/TE = 1000/11.5ms, matrix size = 120 x 58, FOV = 15.84 x 7.65 mm2 and 18 0.5-mm-thick slices. To investigate the ACC role in somatosensation, ACC was inhibited simultaneously with whisker-pad stimulation. Whisker-pad was stimulated electrically with 0.4mA at 4Hz. Mice were stimulated in block design of 40s baseline - 20s stimulation - 60s interstimulus interval - 20s stimulation - 60s recovery. Images were preprocessed and analyzed via GLM. BOLD quantification was performed by calculating the average area-under-curve (AUC) of the two response peaks.Results
Optogenetic activation of inhibitory neurons in the ACC did not affect the thermal pain threshold (Figure 1B) or the capsaicin-induced spontaneous pain behavior (Figure 1Ca). However, ACC inhibition reversed the maintenance of pain hypersensitivity induced by capsaicin or CFA (Figure 1Cb,D). ACC inhibition in both naive and CFA-model mice produced negative BOLD response in the ACC, mediodorsal thalamus (MD), prelimbic (PrL), primary somatosensory hindlimb and barrel cortex (S1HL/S1BF), dorsomedial and dorsolateral/lateral periaqueductal gray (dm and dl/l PAG), superior colliculus (SC), and anteromedial thalamus (AM) (Figure 2A,B). The CFA model resulted in greater negative BOLD response in ACC, S1HL, dmPAG, dl/lPAG, and AM (Figure 2C). Whisker-pad (WP) stimulation resulted in BOLD response in the contralateral S1BF, secondary somatosensory cortex (S2), ventral posterior thalamus (VP), posterior thalamus (PO), dl/lPAG, SC, and secondary motor cortex (M2). When combined with simultaneous ACC inhibition (WPOG), significant decrease in BOLD response was observed in MD, dl/lPAG, and M2. Although non-significant, a modulatory effect is seen in S1BF, PO, and SC as well.Discussion
The ACC is crucially involved in pain perception. In both human and animal studies, activity in the ACC correlated with the intensity of acute pain stimuli3,4. Chronic persistent pain, such as our CFA model, caused hyperactivity in excitatory pyramidal neurons in the ACC. These findings are in support of our fMRI findings as we report greater negative BOLD response in the ACC and other regions in our CFA model. The greater negative BOLD response suggests the baseline neural activity was higher in those areas which suggests chronic pain leads to increased activity in the ACC and its projections related to chronic pain. Interestingly, we also saw S1HL area present with a larger response in the CFA model as this region corresponds to the contralateral hindpaw in which CFA was injected. This suggests chronic pain may involve the primary sensory cortices. Our whisker-pad-optogenetic results suggest ACC modulates dl/lPAG and motor cortex during nociceptive stimulation. Although not significant, a modulatory effect is also seen in the S1BF, S2, and PO, but not VP which suggests ACC may modulate higher-order sensory information rather than ascending sensory information. This coincides with our behavior result in which ACC inhibition did not affect general nociception.Conclusion
We have shown ultrahigh field fMRI with optogenetics may map downstream circuits related to pain hypersensitivity and by utilizing transgenic and pain models, show areas other than ACC or PAG to be involved for future investigations.Acknowledgements
This research was supported by the Institute for Basic Science (IBS-R015-D1)References
- KL., M. R. C. 1968. Sensory, motivational, and central control determinants of pain: a new conceptual model. . In: Kenshalo D, editor. The skin senses. Springfield: C.C. Thomas,, 423-439.
- You, T., Im, G.H. & Kim, SG. Characterization of brain-wide somatosensory BOLD fMRI in mice under dexmedetomidine/isoflurane and ketamine/xylazine. Sci Rep 11, 13110 (2021).
- ZHAO, R., ZHOU, H., HUANG, L., XIE, Z., WANG, J., GAN, W. B. & YANG, G. 2018. Neuropathic Pain Causes Pyramidal Neuronal Hyperactivity in the Anterior Cingulate Cortex. Front Cell Neurosci, 12, 107.
- BUCHEL, C., BORNHOVD, K., QUANTE, M., GLAUCHE, V., BROMM, B. & WEILLER, C. 2002. Dissociable neural responses related to pain intensity, stimulus intensity, and stimulus awareness within the anterior cingulate cortex: a parametric single-trial laser functional magnetic resonance imaging study. J Neurosci, 22, 970-6.
Figures
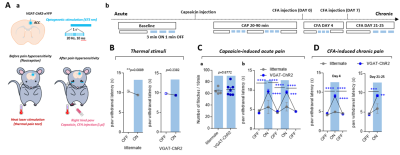
Effects of optogenetic silencing of ACC on
pain-related behavior. A. Schematic design for the experiment (a) and experimental
schedule (b). Silencing of ACC was caused by interneuron activation of
VGAT-ChR2 mice. B-D. The effect of ACC silencing on paw withdrawal latency for
thermal stimuli (B), capsaicin-induced acute pain (C), and CFA-induced chronic
pain (D)
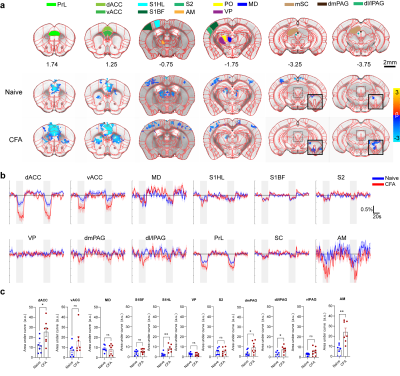
BOLD response to ACC inhibition in naïve and CFA
models.
A. ROI definitions and BOLD response map p=0.05 corrected.
Insets for the last two slices show a lower threshold for PAG activation. B. Percent
signal change of active areas. VP, ventral posterior thalamus shown for control. C. AUC
quantification of BOLD amplitude. *0.05, **0.01
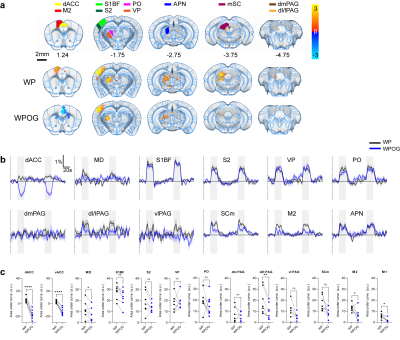
BOLD response to ACC inhibition during whisker-pad
stimulation.
A. ROI definitions and BOLD response p=0.05
corrected. WP, whisker-pad only stimulation; WPOG, whisker-pad and optogenetic
stimulation. M2, secondary motor cortex; APN, anterior pretectal nucleus. B.
Percent signal change of active areas. C. AUC quantification of BOLD
amplitudes. *0.05, **0.01, ***0.001,
DOI: https://doi.org/10.58530/2022/4674