4673
Investigation of Pulmonary Physiological Compensatory Mechanism with Hyperpolarized 129Xe MR1Key Laboratory of Magnetic Resonance in Biological Systems, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, National Center for Magnetic Resonance in Wuhan, Wuhan Institute of Physics and Mathematics, Innovation Academy for Precision Measurement Science and Technology, Chinese Academy of Sciences- Wuhan National Laboratory for Optoelectronics, Wuhan, China
Synopsis
In this study, we tried to use hyperpolarized (HP) 129Xe MR to explore pulmonary physiological compensatory mechanisms. With the increased Xe washout times, obvious changes of chemical shift, FWHM, RBC oscillation amplitude and the parameters extracted from MOXE model could be found. Our study demonstrates the feasibility of HP 129Xe MR for assessing pulmonary compensatory capacity, which would be helpful for understanding lung function changes caused by pulmonary diseases and early diagnosis.
Introduction
By using chemical shift saturation recovery (CSSR) spectroscopy and air-blood exchange model (such as MOXE),1 impaired air-blood exchange function caused by lung diseases could be evaluated by using HP 129Xe MR.2 However, hypoxia could cause physiological compensatory responses in the body, and whether such responses could be detected by CSSR is worth studying. Although previous studies have demonstrated the chemical shift and full width at half maximum (FWHM) of 129Xe could be affected by oxygen concetration,3 the chemical shift and FWHM changes in animal are still needed to be systematically studied considering species differences.4 Previous studies have reported HP 129Xe MR could detect RBC signal oscillations associated with cardiac cycle.5 However, some pulmonary physiological compensatory mechanisms may affect the amplitude of RBC oscillations. In this study, we tried to evaluate theses parameters under acute hypoxia challenge to investigate pulmonary physiological compensatory mechanism.Methods
A total of 40 Sprague-Dawley rats were divided into two groups (30 in experimental group, 10 in control group). The experimental group was treated with monocrotaline (60 mg/kg body weight, intraperitoneal injection) to establish a pulmonary arterial hypertension (PAH) model6. HP 129Xe MR measurement was conducted on day 7, 14 and 21 (10 rats in each time point, except for 3 rats, for the data of whom were too poor to be analyzed) after treatment. The control group were treated with normal saline (60 mg/kg body weight, intraperitoneal injection), and HP 129Xe MR measurement was conducted on day 22 after treatment. All the MR experiments were performed on a 7.0 T animal MRI scanner (Bruker Biospec 70/20 USR; Germany), and rats were ventilated using a home-built HP gas delivery system. At baseline, rats were ventilated high purity oxygen firstly, and then oxygen in the lung was washed out using 8 consecutives breaths with pure HP Xe during HP Xe data collection. The time for each Xe breath hold is 5 seconds. The experiment was repeated 6 times (3 CSSR and 3 constant TR spectroscopy) for each rat after the blood oxygen saturation recovered. For CSSR experiments, 24 exchange time points ranging from 2 ~ 400 ms were used.7 For constant TR spectroscopy, TR = 50 ms, flip angle = 20°, and the number of repetitions = 50 in each breath. Spectrum analysis was performed using MATLAB 2021a. The parameters including area, frequency, FWHM, and phase were extracted by peak fitting.8 And then, we can obtain RBC oscillation amplitude,9 and lung physiological parameters by fitting a sine or a MOXE model.1Results
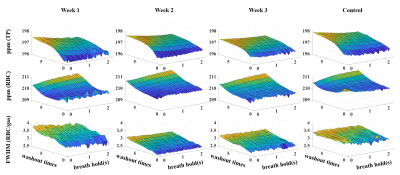
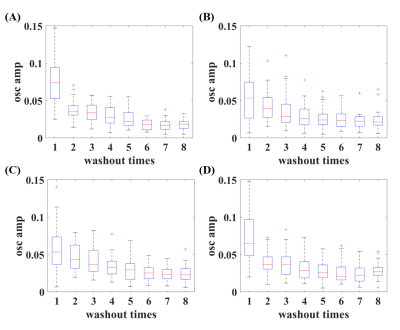
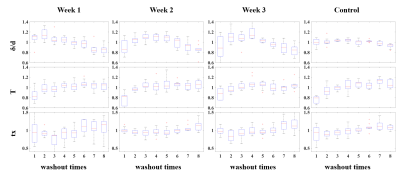
The mean ppm (TP), ppm (RBC) and FWHM (RBC/gas) were shown in Figure 1. Xe washout times and breath-hold time will affect these parameters, and these effects are modified by disease severities. Box plot of RBC oscillation amplitudes were shown in Figure 2. With the increased Xe washout times, RBC oscillation amplitude decreased. Box plot of pulmonary physiological parameters, which were normalized by the mean of this parameter each rat, were shown in Figure 3. With the Xe washout times increasing, δ/d increases firstly and then decreases, while tx decreases firstly and then increases. T has an obvious rising trend.Discussion and Conclusion
In this study, we explored pulmonary physiological compensatory mechanism by analyzing the trend of chemical shift, FWHM, RBC oscillation amplitude and lung physiological parameters with HP 129Xe MR. The chemical shift change trends of RBC and TP are obviously different with that in human, and this is probably caused by the species differences.3,4 Disease severities could also affect chemical shift and its change trend,10 which may be used for evaluating physiological compensatory capacity difference. RBC oscillation amplitude was negative correlated with Xe washout times, which might be contributed to the capillary bed increase in pulmonary vascular during hypoxia.11 Changes of capillary bed volume can also explain the change of T. HPR mechanism can explain why δ/d increases and tx decreases in the first several washout times. Our preliminary results indicated HP Xe MR has potential in quantifying the physiological compensatory capacity changes caused by lung diseases.Acknowledgements
This work is supported by National Natural Science Foundation of China (91859206, 21921004, 81825012), National key Research and Development Project of China (2018YFA0704000), Key Research Program of Frontier Sciences (ZDBS-LY-JSC004) and Scientific Instrument Developing Project of the Chinese Academy of Sciences (GJJSTD20200002, YJKYYQ20200067), CAS. Haidong Li acknowledges the support from Youth Innovation Promotion Association, CAS (2020330). Xin Zhou acknowledges the support from the Tencent Foundation through the XPLORER PRIZE.References
1. Chang, Y.V. MOXE: a model of gas exchange for hyperpolarized 129Xe magnetic resonance of the lung. Magn Reson Med 69, 884-890 (2013).
2. Li, H., et al. Damaged lung gas exchange function of discharged COVID-19 patients detected by hyperpolarized (129)Xe MRI. Sci Adv 7(2021).
3. Norquay, G., Leung, G., Stewart, N.J., Wolber, J. & Wild, J.M. (129) Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized (129) Xe NMR. Magn Reson Med 77, 1399-1408 (2017).
4. Friedlander, Y., et al. Effect of inhaled oxygen concentration on (129) Xe chemical shift of red blood cells in rat lungs. Magn Reson Med 86, 1187-1193 (2021).
5. Ruppert, K., et al. Detecting pulmonary capillary blood pulsations using hyperpolarized xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magn Reson Med 75, 1771-1780 (2016). 6. 6. Virgincar, R.S., et al. Quantitative (129)Xe MRI detects early impairment of gas-exchange in a rat model of pulmonary hypertension. Sci Rep 10, 7385 (2020).
7. Zhang, M., et al. Quantitative evaluation of lung injury caused by PM2.5 using hyperpolarized gas magnetic resonance. Magn Reson Med 84, 569-578 (2020).
8. Chang, Y.V., et al. Quantification of human lung structure and physiology using hyperpolarized 129Xe. Magn Reson Med 71, 339-344 (2014).
9. Niedbalski, P.J., et al. Mapping cardiopulmonary dynamics within the microvasculature of the lungs using dissolved (129)Xe MRI. J Appl Physiol (1985) 129, 218-229 (2020).
10. Friedlander, Y., et al. Chemical shift of (129) Xe dissolved in red blood cells: Application to a rat model of bronchopulmonary dysplasia. Magn Reson Med 84, 52-60 (2020).
11. Zavorsky, G.S., Walley, K.R. & Russell, J.A. Red cell pulmonary transit times through the healthy human lung. Exp Physiol 88, 191-200 (2003).
Figures