4671
Assessment of intra-tumoral heterogeneity of prostate cancer using Intravoxel Incoherent Motion (IVIM)1Institute of Medical Physics, The University of Sydney, Sydney, Australia, 2Department of Radiation Oncology, Calvary Mater Hospital, Waratah, Australia, 3South Western Sydney Clinical School, University of New South Wales, Sydney, Australia, 4Liverpool and Macarthur Cancer Therapy Centre, Liverpool, Australia, 5Ingham Institute of Applied Medical Research, Liverpool, Australia, 6Faculty of Medicine and Health, The University of Sydney, Sydney, Australia, 7Department of Radiology, Westmead Hospital, Westmead, Australia
Synopsis
Mapping the intra-tumoral heterogeneity of perfusion before and after treatment offers the opportunity to identify hypoxic regions in the tumour and predict recurrent disease. Intravoxel Incoherent Motion (IVIM) parametric maps were obtained in patients with prostate cancer before and after radiation therapy. IVIM shows potential for the assessment of intra-tumoral heterogeneity of perfusion in prostate cancer.
Introduction
Hypoxia has been linked to treatment resistance, tumour recurrence and poor patient outcomes after radiation therapy (RT) for prostate cancer (PCa)(1). Tumours containing hypoxic regions will have variable perfusion which would be expected to normalise following RT. Hence mapping the intra-tumoral heterogeneity of perfusion before and after treatment has the potential to predict recurrent disease. Dynamic contrast enhanced (DCE) MRI is routinely used to assess tumour perfusion in PCa(2). Intravoxel Incoherent Motion (IVIM) has recently emerged as a possible alternative perfusion imaging technique for PCa(3). The potential for IVIM to monitor changes in PCa perfusion as a response to RT has been demonstrated(4), however, reporting of intra-tumoral heterogeneity of IVIM parameters in PCa is limited. Here, we present an analysis of the spatial heterogeneity of IVIM parameters in PCa before and after radiation therapy.Methods
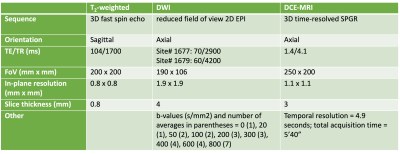
A longitudinal study including 21 participants with localised PCa was performed to study RT treatment response using multi-parametric MRI (SI-BiRT clinical trial: ANZCTR UTN U1111-1221-9589). Androgen deprivation therapy (ADT) was given to 14 patients prior to RT. T2-weighted, DWI (pre-contrast) and DCE-MRI imaging was performed at up to 4 timepoints (pre-ADT, pre-RT, and 6- and 12-months post-RT) with scan parameters detailed in Table 1.DCE-MRI signals were fitted to a Tofts model using vendor-supplied inline fitting software to generate the transfer rate constant (Ktrans) and extracellular volume fraction (ve) parametric maps. DWI images were denoised using a non-local means filter from the DIPY package(5). IVIM parametric maps were calculated in two stages, performing a mono-exponential fit with b ≥ 200 s/mm2 to estimate the diffusion coefficient (D), followed by a bi-exponential fit to estimate the perfusion fraction (f)(6).
Parametric maps were rigidly registered to the corresponding T2-weighted images. Tumours were automatically delineated on pre-treatment T2-weighted images using an inhouse developed deep learning model(7). T2-weighted images of subsequent scans were registered to the baseline using the Elastix module implemented in 3DSlicer(8). The deformable transformations were applied to the parametric maps and the delineations were copied.
Median and inter-quartile range (IQR) of D, f, Ktrans and ve were obtained within the delineations. Correlation between IVIM and DCE-MRI parameters was assessed using the Pearson’s correlation coefficient. Intra-tumoral heterogeneity of the parameters was quantified as $$$100×\frac{IQR}{median}$$$.
Results
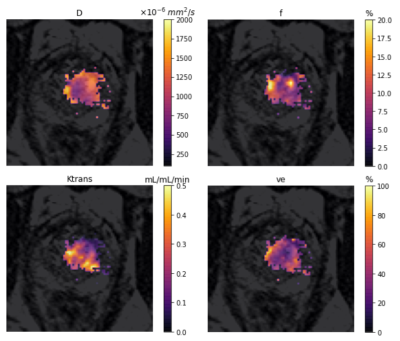
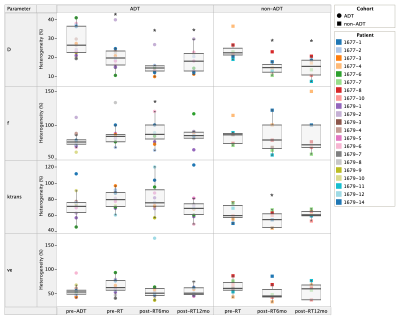
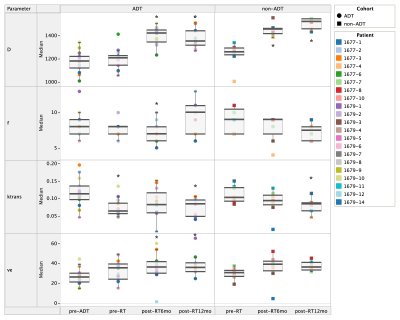
Figure 1 shows the intra-tumoral heterogeneity in IVIM and DCE-MRI parametric maps for a single patient pre-treatment. Change in intra-tumoral heterogeneity of all parameters as a response to treatment is shown in Figure 2. Among the IVIM parameters, a significant decrease in tumour heterogeneity was observed in D after commencement of ADT, and at 6-months post-RT. An increase in heterogeneity of f was also observed, but only in the ADT cohort. Neither of the DCE-MRI parameters demonstrated any change in heterogeneity. A decrease in Ktrans in the non-ADT cohort was observed at 6-months post-RT.Figure 3 shows the median values of tumour IVIM and DCE-MRI parameters at all timepoints. Among the IVIM parameters, a significant increase in D was observed at 6-months post-RT in both the ADT and non-ADT cohorts. f was found to decrease significantly at this timepoint in the ADT cohort, suggesting minimal effect of RT on tumour perfusion. Significant changes in the DCE-MRI parameters as a response to treatment were also observed. Ktrans decreased significantly immediately after ADT commencement and was found to be significantly reduced by 12-months post-RT in both cohorts. ve correspondingly increased during this time, however, a significant change was only observed in the ADT cohort.
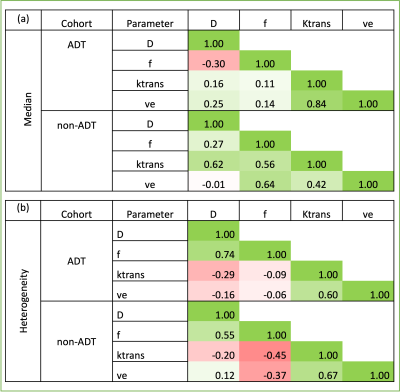
Table 2 shows the pair-wise correlation coefficients between median and heterogeneity of IVIM and DCE parameters. Low to no correlation was observed between the parameters in the ADT cohort. However, in the non-ADT cohort, moderate positive correlations of median D with Ktrans, and of f with Ktrans and ve were observed. A moderate negative correlation was also found between intra-tumoral heterogeneity of f with Ktrans and ve.
Discussion
Previous studies have reported on the potential for IVIM to monitor changes in PCa perfusion in treatment response studies, as an alternative to DCE-MRI(4). In this study, moderate correlation between IVIM and DCE-MRI parameters was only observed in the non-ADT patient cohort, suggesting further investigation of the effects of ADT on the parameters is required. However, a general decrease in f and Ktrans, accompanied by an increase in ve and D before and after treatment was seen, which corresponds to the expected slowdown of new blood vessel formation and cell growth.All IVIM and DCE-MRI parameters demonstrated intra-tumoral heterogeneity pre-treatment as well as changes in heterogeneity as a response to treatment. D was least sensitive to tumour heterogeneity but demonstrated the strongest treatment response. Larger heterogeneity was observed in the other parameters, however, this may have been due to the low precision of the model fitting methods.
Conclusion
Pre-treatment intra-tumoral heterogeneity was observed in IVIM and DCE-MRI parameter maps demonstrating the potential to identify hypoxic sub-volumes. Changes in heterogeneity were observed following treatment indicating the potential for IVIM to predict persistent hypoxia and provide an alternative to DCE-MRI in longitudinal treatment response studies. Further refinement of the IVIM parametric mapping methods is required to improve confidence in predicting response to treatment.Acknowledgements
We thank Dr. Faisal Mahmood (University of Southern Denmark) for valuable discussions on the post-processing methods. We thank Dr. Amy Hayden, Dr. Sandra Turner, Dr. Viet Do and Dr. Jarad Martin for their support in the design and conduct of the SI-BiRT clinical trial. We are also grateful to Siemens Healthineers for their support.References
1. Milosevic M, Warde P, Menard C et al. Tumor hypoxia predicts biochemical failure following radiotherapy for clinically localized prostate cancer. Clin Cancer Res 2012; 18(7):2108-2114.
2. Berman RM, Brown AM, Chang SD et al. DCE MRI of prostate cancer. Abdom Radiol 2016; 41(5):844-853.
3. Valerio M, Zini C, Fierro D et al. 3T multiparametric MRI of the prostate: Does intravoxel incoherent motion diffusion imaging have a role in the detection and stratification of prostate cancer in the peripheral zone? Eur J Radio 2016; 85(4):790-794
4. Kooreman ES, van Houdt PJ, Keesman R et al. Daily Intravoxel Incoherent Motion (IVIM) In Prostate Cancer Patients During MR-Guided Radiotherapy—A Multicenter Study. Front Oncol 2021; 11(705964):1-9.
5. Garyfallidis E, Brett M, Amirbekian B et al. DIPY, a library for the analysis of diffusion MRI data. Frontiers in Neuroinformatics 2014; 8(8):1-17.
6. Jalnefjord O, Andersson M, Montelius M et al. Comparison of methods for estimation of the intravoxel incoherent motion (IVIM) diffusion coefficient (D) and perfusion fraction (f). MAGMA. 2018;31(6):715-723.
7. Sun Y. https://github.com/sunyu0410/prostate-mpmri
8. Klein S, Staring M, Murphy Ket al. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29(1):196-205.
Figures