4668
Optogenetically-evoked Brain-wide Spindles Alleviate Associative Memory Dysfunction in Aging Animal1Laboratory of Biomedical Imaging and Signal Processing, The University of Hong Kong, Hong Kong SAR, China, 2Department of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong SAR, China, 3School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong SAR, China
Synopsis
Memory consolidation, the ability to transform new memory into long-term knowledge, declines with age. Impairment of thalamo-cortical spindle activities at systems level has been proposed as a mechanism underlying such memory dysfunction. However, it remains unknown whether targeted neuromodulation of spindle activities can arrest decline of memory consolidation functions in aging brains. Here, we demonstrate in an accelerated aging animal model that optogenetically-evoked spindle activities from the somatosensory thalamus alleviate associative memory consolidation dysfunction through potentiating brain-wide sensorimotor and limbic regions. Our work provides valuable insights into the therapeutic implications of targeted spindle manipulation to rescue age-related memory consolidation declines.
Purpose
One grand challenge for modern society is the rapid growth of aging population1. The decline of learning and memory capacity due to aging can severely affect quality of life1,2. Impairment of neural oscillatory activities is widely considered to underlie such memory deficits3-6. In particular, thalamo-cortical spindle oscillatory activities7,8 were found to decrease with age in their density, amplitude and duration4,9-11 that correlated with learning and memory consolidation deficits4,10,11. Further, some indirect spindle manipulation studies (e.g., manipulating slow oscillations with subsequent non-specific effects on spindles) implicated potential therapeutic interventions to rescue such age-related memory deficits12,13. However, these studies were limited by the lack of an integrative platform that allows the identification of specific spindle activities in action, their brain-wide targets, and actions supporting memory consolidation. Consequently, whether and where elevating specific spindle activities may alleviate memory functional deficits remain unknown. Previously, through combining spatiotemporally-precise optogenetic stimulation, fMRI, and behavioral tests in normal rats, we successfully revealed the brain-wide propagation targets of thalamic-evoked spindle activities and their critical sites of action for facilitating memory consolidation14,15. Here, we employ this integrative neuroimaging approach to further investigate the therapeutic effects of optogenetically-evoked spindle activities on memory consolidation dysfunction in rat aging model.Methods
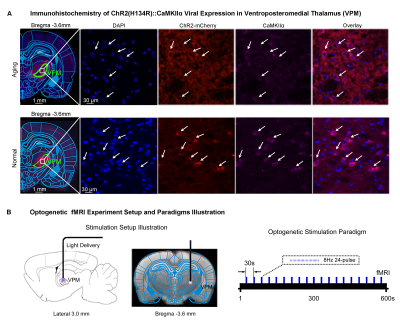
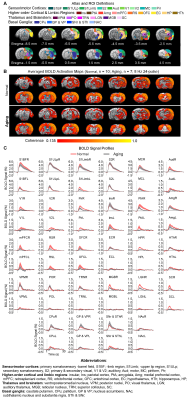
Animal preparation and MRI experimental setup: 3μl AAV5-CaMKIIα::ChR2(H134R)-mCherry was injected to somatosensory-specific ventroposteromedial (VPM) thalamus of adult SD rats (6 weeks). After 4 weeks, optogenetically transfected animals were injected with D-galactose (50mg/kg, subcutaneous) daily for 8 weeks to induce accelerated aging16 in the animals. Opaque optical fiber cannulas were then implanted at the injection sites (Figure 1A). fMRI experiments were performed at 7T scanner under 1.0% isoflurane.Optogenetic fMRI experiments: 8Hz 24-pulses blue (473nm) light optogenetic stimulation coinciding with typical electrophysiological characteristics of spontaneous spindle activities7,8,14,15 were delivered once every 30s (10ms pulse width, 40mW/mm2; Figure 1B). Coherence analysis17 was applied to identify significant BOLD responses to optogenetic stimulation and BOLD profiles were extracted from atlas-based ROIs (Figure 2).
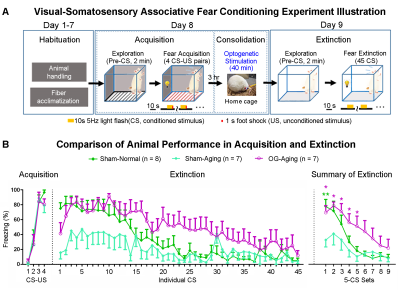
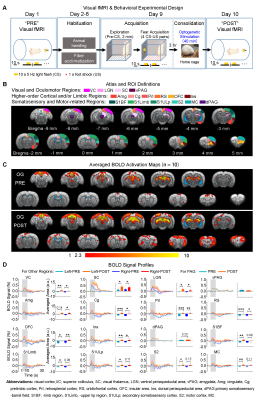
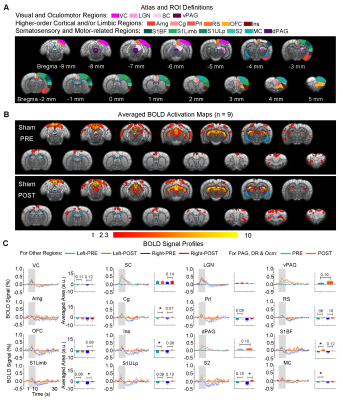
Visual fMRI and behavior experiments: Optogenetic (OG) and Sham animals acquired visual-somatosensory associative memory via receiving pairs of 10s 5Hz light flash (CS, conditioned stimulus) co-terminated with foot shock (US, unconditioned stimulus) (Figures 3A). Animals received 40min of 8Hz 24-pulse VPM stimulation during memory consolidation phase. Memory performance was then assessed by measuring animal freezing levels against the 45 repetitions of CS (Figure 3B). Visual fMRI (vfMRI) experiments using visual stimulation identical to the CS were conducted 8 days before (PRE) and 1 day after (POST) animals underwent the aforementioned behavioral training (Figure 4A). BOLD responses were mapped via a GLM model.
Results
fMRI reveals similar brain-wide propagation targets for thalamic-evoked spindle activities in aging and normal animals (Figure 2): Optogenetically initiated thalamic spindle activities in aging animals evoked robust BOLD activations across brain-wide regions as in normal animals, including sensorimotor cortical and thalamic nuclei (i.e., somatosensory, visual and auditory), brainstem and basal ganglia, and non-sensorimotor limbic regions such as amygdala and the hippocampal formation.Optogenetically-evoked brain-wide spindle activities alleviate the decline of memory consolidation functions in aging animals (Figure 3): After 24 hours, visual-somatosensory associative memory in Sham-Aging animals decreased significantly compared to the Sham-Normal animals, demonstrating impaired memory consolidation functions in the accelerated aging animal model. Notably, evoking brain-wide spindle activities in OG-Aging animals rescued such decreased memory functions to a level similar to Sham-Normal animals.
Potentiation of brain-wide sensorimotor and limbic regions underlies enhanced associative memory consolidation in aging animals: We found that after optogenetic stimulation, OG-aging group (POST) showed significantly increased visual cortical, thalamic and brainstem activations compared to baseline (PRE) and displayed additional activations in amygdala, insular area and various somatosensory, motor, frontal (cingulate, prelimbic and orbitofrontal) and retrosplenial cortical regions (Figure 4). Without optogenetic stimulation, the Sham-aging group (POST) only showed significantly increased BOLD activations in cingulate, insular, somatosensory, and motor cortices (Figure 5).
Discussion and Conclusion
Our study demonstrates that optogenetically-evoked spindle activities enhanced visual-somatosensory associative memory consolidation in accelerated aging animals, specifically through response potentiation in brain-wide sensorimotor and limbic regions. We propose that sensorimotor regions may subserve sensorimotor modality-specific memory trace reactivations while higher-order cortical and limbic regions support integration of reactivated information and consolidation of cross-modal associations18-21. Sham-Aging animals showed response potentiation in limited somatosensory, motor and limbic regions, which likely reflected the action of spontaneous spindle activities in memory consolidation. However, in OG-Aging animals, spindle activities were increased across their brain-wide propagation targets, leading to more regions being potentiated compared to Sham-Aging animals. We noticed that although optogenetically-evoked spindle activities in aging animals could propagate to brain-wide targets as those in normal animals, aging led to overall decreased spindle propagation to multiple remote regions. This indicates that topographically specific declines of spindle activities propagation in aging brains mainly occur in regions beyond the circuits of spindle initiation. Compared to normal animals14, dorsal periaqueductal gray were not potentiated after stimulation, likely due to such decreased propagation of spindle activities in aging brains. Nevertheless, optogenetically-evoked spindle activities were still able to potentiate brain-wide regions that facilitate associative memory consolidation in aging animals. In conclusion, this study reveals that targeted initiation of brain-wide spindle propagation at somatosensory thalamus can alleviate aging-associated memory consolidation deficit through large-scale multi-target potentiation.Acknowledgements
This work was supported in part by Hong Kong Research Grant Council (R7003-19F, HKU17112120 and HKU17127121 to E.X.W., and HKU17103819, HKU17104020 and HKU17127021 to A.T.L.L.), Lam Woo Foundation, and Guangdong Key Technologies for Treatment of Brain Disorders (2018B030332001) and Guangdong Key Technologies for Alzheimer’s Disease Diagnosis and Treatment (2018B030336001) to E.X.W.References
1. Organization, W.H. Decade of healthy ageing: baseline report. (2020).
2. Grady, C. The cognitive neuroscience of ageing. Nat Rev Neurosci 13, 491-505 (2012).
3. Mander, B.A., Winer, J.R. & Walker, M.P. Sleep and Human Aging. Neuron 94, 19-36 (2017).
4. Helfrich, R.F., Mander, B.A., Jagust, W.J., Knight, R.T. & Walker, M.P. Old Brains Come Uncoupled in Sleep: Slow Wave-Spindle Synchrony, Brain Atrophy, and Forgetting. Neuron 97, 221-230 e224 (2018).
5. Djonlagic, I., Mariani, S., Fitzpatrick, A.L., Van Der Klei, V., Johnson, D.A., Wood, A.C., Seeman, T., Nguyen, H.T., Prerau, M.J., Luchsinger, J.A., Dzierzewski, J.M., Rapp, S.R., Tranah, G.J., Yaffe, K., Burdick, K.E., Stone, K.L., Redline, S. & Purcell, S.M. Macro and micro sleep architecture and cognitive performance in older adults. Nat Hum Behav 5, 123-145 (2021).
6. Mander, B.A., Marks, S.M., Vogel, J.W., Rao, V., Lu, B., Saletin, J.M., Ancoli-Israel, S., Jagust, W.J. & Walker, M.P. beta-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci 18, 1051-1057 (2015).
7. Fernandez, L.M.J. & Luthi, A. Sleep Spindles: Mechanisms and Functions. Physiol Rev 100, 805-868 (2020).
8. Warby, S.C., Wendt, S.L., Welinder, P., Munk, E.G., Carrillo, O., Sorensen, H.B., Jennum, P., Peppard, P.E., Perona, P. & Mignot, E. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods 11, 385-392 (2014).
9. Purcell, S.M., Manoach, D.S., Demanuele, C., Cade, B.E., Mariani, S., Cox, R., Panagiotaropoulou, G., Saxena, R., Pan, J.Q., Smoller, J.W., Redline, S. & Stickgold, R. Characterizing sleep spindles in 11,630 individuals from the National Sleep Research Resource. Nat Commun 8, 15930 (2017).
10. Mander, B.A., Rao, V., Lu, B., Saletin, J.M., Ancoli-Israel, S., Jagust, W.J. & Walker, M.P. Impaired prefrontal sleep spindle regulation of hippocampal-dependent learning in older adults. Cereb Cortex 24, 3301-3309 (2014).
11. Fogel, S.M., Albouy, G., Vien, C., Popovicci, R., King, B.R., Hoge, R., Jbabdi, S., Benali, H., Karni, A., Maquet, P., Carrier, J. & Doyon, J. fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 35, 3625-3645 (2014).
12. Ladenbauer, J., Ladenbauer, J., Kulzow, N., de Boor, R., Avramova, E., Grittner, U. & Floel, A. Promoting Sleep Oscillations and Their Functional Coupling by Transcranial Stimulation Enhances Memory Consolidation in Mild Cognitive Impairment. J Neurosci 37, 7111-7124 (2017).
13. Ladenbauer, J., Külzow, N., Passmann, S., Antonenko, D., Grittner, U., Tamm, S. & Flöel, A. Brain stimulation during an afternoon nap boosts slow oscillatory activity and memory consolidation in older adults. NeuroImage 142, 311-323 (2016).
14. Wang, X., Leong, A.T.L., Tan, S., Ma, T., Khong, P., Lim, L. & Wu, E. Functional MRI investigation of Optogenetically-evoked Spindle-like Neural Activity and Memory Consolidation. in Proceedings of International Society of Magnetic Resonance in Medicine, Virtual Conference, p1353 (2020).
15. Wang, X., Leong, A.T.L., Guo, A.S., Dong, C.M. & Wu, E.X. Optogenetically-evoked spindle-like activity from thalamus propagates brain-wide and enhances rsfMRI connectivity. in Proceedings of International Society of Magnetic Resonance in Medicine, Montreal, Canada, p3752 (2019).
16. Ali, T., Badshah, H., Kim, T.H. & Kim, M.O. Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-KB/JNK signaling pathway in aging mouse model. J Pineal Res 58, 71-85 (2015).
17. Lee, J.H., Durand, R., Gradinaru, V., Zhang, F., Goshen, I., Kim, D.S., Fenno, L.E., Ramakrishnan, C. & Deisseroth, K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788-792 (2010).
18. Dudai, Y., Karni, A. & Born, J. The consolidation and transformation of memory. Neuron 88, 20-32 (2015).
19. Antony, J.W., Schonauer, M., Staresina, B.P. & Cairney, S.A. Sleep Spindles and Memory Reprocessing. Trends Neurosci 42, 1-3 (2019).
20. Diekelmann, S. & Born, J. The memory function of sleep. Nat Rev Neurosci 11, 114 (2010). 21. Klinzing, J.G., Niethard, N. & Born, J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22, 1598-1610 (2019).
Figures