4665
Radiomics Analysis Based on IVIM-DWI for Early Assessment of Transplanted Kidney Dysfunction1Department of Radiology, Tianjin First Central Hospital, Tianjin, China, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 3MR Collaboration, Siemens Healthcare Ltd., Beijing, China
Synopsis
This study investigated the feasibility of predicting early renal function impairment after renal transplantation based on an intravoxel incoherent motion imaging (IVIM)diffusion weighted imaging (DWI)radiomics model. For noninvasive detection of kidney function impairment at an early stage, IVIM-DWI applies a bi-exponential model to evaluate both capillary perfusion and tissue diffusion. The overall accuracy of the radiomics model was 79.3%, with 73.3% sensitivity and 85.7% specificity in distinguishing renal function impairment after renal transplantation. These results demonstrate the potential of a radiomics model for a reliable non-invasive diagnosis of renal transplant function.
Introduction
Renal transplantation is an important treatment for patients with end-stage renal disease. In recent years, due to the continuous development of surgical techniques, immunosuppressants and postoperative monitoring measures, the long-term survival rate of renal transplantation has been greatly improved; however, the incidence of different degrees of graft function injury and even complete loss of function is still high[1]. Therefore, the early diagnosis and noninvasive monitoring of renal allograft function injury is an urgent clinical problem to be solved. The clinical application of renal biopsy in monitoring renal allograft function is limited because of its invasiveness and low specificity of serum creatinine[2].Radiomics can obtain various implicit information closely related to disease occurrence development and prognosis[3]. The purpose of this study was to develop a radiomics model based on IVIM-DWI images for early assessment of transplanted kidney dysfunction, with the goal to provide valuable information for early clinical diagnosis and noninvasive monitoring of renal function impairment.Methods
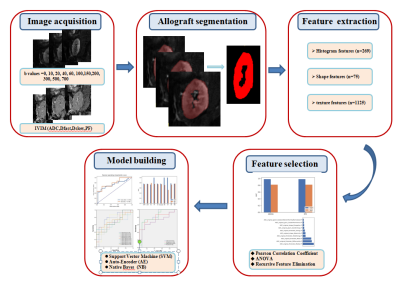
A total of 97 participants were enrolled(72 male,27 female; mean age 36.77±10.71years). Recipients were divided into two groups with either normal or impaired function according to the estimated glomerular filtration rate (eGFR) with a threshold of 60 ml/min/1.73 m2. Patients were randomly assigned to either a training cohort (n= 68) or a validation cohort (n= 29). All MR examinations were performed on a 3T system (MAGNETOM Trio a Tim System, Siemens Healthcare, Erlangen, Germany). IVIM-DWI was acquired using a prototype single-shot echo planar imaging (EPI) sequence with 11 b-values (0, 10, 20, 40, 60, 100, 150, 200, 300, 500, and 700 s/mm2). Apparent diffusion coefficient (ADC) maps were generated inline after data acquisition. IVIM-derived perfusion fraction (PF), pseudo-diffusion coefficient (DFast), and standard diffusion coefficient (DSlow) parametric maps were generated using a prototype postprocessing software (MR Body Diffusion Toolbox, Siemens). Figure 1 illustrates the processing pipeline. Whole-kidney segmentation was performed on eachb-value image, ADC map, and IVIM-DWI derived parametric map using ITK-SNAP (v3.6.0, http://www.itksnap.org/). The volumes of interest (VOIs) were manually delineated along the inner edge of the transplanted kidney parenchyma on all slices. A total of 1604 radiomics features were then extracted from each b-value image and parametric map using FeAtureExplorer Pro(FAE Pro, v0.3.7, https://github.com/salan668/FAE.git).Mean normalization was applied on the feature matrix. Pearson Correlation Coefficient (PCC) was used to exclude redundant features. Analysis of Variance (ANOVA), relief, and Recursive Feature Elimination (RFE) were chosen for feature selection.Support Vector Machine (SVM),Linear Discriminative Analysis (LDA), auto-Encoder(AE), Random Forest(RF),Logistical Regression (LR),Least Absolute Shrinkage and Selection Operator (LASSO), Adaboost(AB), Decision Tree(DT), Gaussian Process(GP), and Naïve Bayes(NB)were applied to construct radiomics models in all b-images and combinatorial parameters. The discriminative performances of the radiomics models were demonstrated by receiveroperator characteristic (ROC) curves. The area under the curve(AUC), accuracy, sensitivity, and specificity of the optimal cutoffvalue were obtained from ROC analysis. The clinicopathologic characteristics and radiologic features in the training and validation cohorts were compared using Student’s t-test orthe Mann–Whitney U-test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables. SPSS software version 23.0 (IBM Corp., Armonk, NY, USA) and Python (Python Software Foundation, version 3.5.2) were used for the statistical analysis.Results
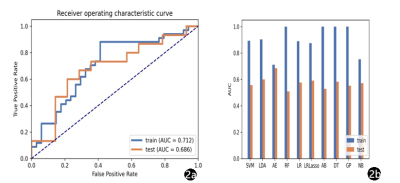
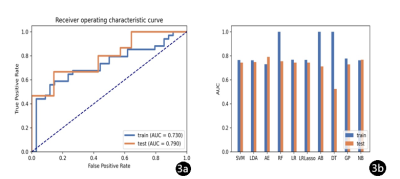
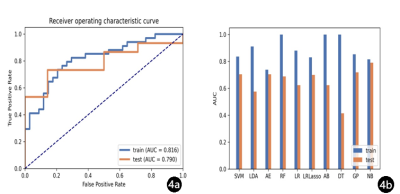
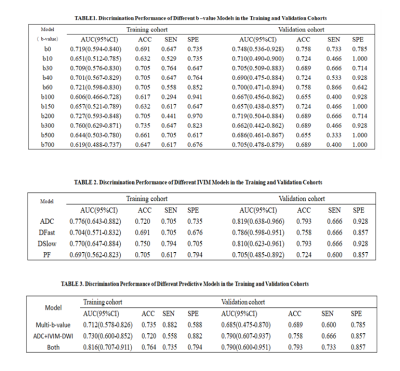
IVIM-DWI illustrated the best performance with an AUC of 0.686 (95% CI: 0.475–0.870), using RFE feature selection and an AE classifier yielded the highest AUC using 18 features(Figure 2). The AUCs of the training and validation datasets achieved 0.712 and 0.686, respectively. IVIM-DWI derived parametric maps showed the best performance with an AUC of 0.790 (95% CI: 0.607–0.937), using ANOVA feature selection and an AE classifier yielded the highest AUC using 3 features (Figure 3). The AUCs of the training and test datasets achieved 0.770 and 0.790, respectively (Table 2). Radiomics models based on the combination of different b-images and parametric maps were built. The b-image and IVIM-DWI derived parametric maps showed the best performance with an AUC of 0.790 (95% CI: 0.600–0.951), using ANOVA feature selection and an NB classifier yielded the highest AUC using 16 features (Figure 4). The AUCs of the training and test datasets achieved 0.816 and 0.790, respectively (Table 3).Discussion and Conclusions
We developed a radiomics model that can be used to predict impaired allograft function after kidney transplantation. This tool may be used to assist clinicians in assessing transplanted kidney function by providing valuable information for early clinical diagnosis and noninvasive monitoring of renal allograft function injury. However, this is a retrospective study, so potential selection bias may be introduced. Secondly, the small sample size, long inclusion period and lack of external verification may affect the robustness of our conclusions. Therefore, more prospective research cohort populations based on multicenter data are needed to verify the performance of the proposed prediction model and better summarize these results.Acknowledgements
We sincerely thank the participants in this study.References
[1] Abdeltawab H, Shehata M, Shalaby A, et al. A Novel CNN-Based CAD System for Early Assessment of Transplanted Kidney Dysfunction. Sci Rep. 2019. 9(1): 5948.
[2] Jiang SH, Karpe KM, Talaulikar GS. Safety and predictors of complications of renal biopsy in the outpatient setting. Clin Nephrol. 2011. 76(6): 464-9.
[3] Koyner JL, Carey KA, Edelson DP, Churpek MM. The Development of a Machine Learning Inpatient Acute Kidney Injury Prediction Model. Crit Care Med. 2018. 46(7): 1070-1077.
Figures