4662
Repeatability and reliability of intravoxel incoherent motion derived parameters in evaluation of liver heterogeneity1Radiology, Guangdong Hospital of Traditional Chinese Medicine, Zhuhai, Zhuhai, China, 2MR Research, GE Healthcare, Beijing, China, Beijing, China, 3Hepatology, Guangdong Hospital of Traditional Chinese Medicine, Zhuhai, Zhuhai, China, 4Laboratory, Guangdong Hospital of Traditional Chinese Medicine, Zhuhai, Zhuhai, China
Synopsis
This study aimed to investigate the repeatability and reliability of IVIM derived parameters in evaluation of liver heterogeneity. Patients with early liver fibrosis and healthy subjects were recruited and underwent MRI using respiratory triggered SS-DWI with 12 b values and an IDEAL-IQ sequence. Two readers independently analyzed liver segment data using mono-exponential, bi-exponential and stretched exponential models. CVs and ICCs were calculated before and after the exclusion of subjects with potential liver fat deposition. ADC, D and α measurements of right liver lobe were satisfactorily repeatable, regardless of fat deposition. Segment-VII and -VIII values may be more repeatable and reliable.
Introduction and Purpose
Diffusion-weighted imaging (DWI) has been explored and applied widely in abdominal and pelvic organs. Intravoxel incoherent motion (IVIM) is a single-shot spin echo–based DWI technique that utilizes multiple b values, enabling the two-component analysis of random water motion in organic tissues and yielding a pure molecular diffusion coefficient (D), perfusion fraction (f), and perfusion-related diffusion coefficient (D*). It has been considered as a potential marker of diffuse organic disease and focal lesions (1,2). Despite the increasing number of applications for the quantitative measurement of liver lesions, parameter values relevant to fibrosis diagnosis and grading are inconsistent (3-5). Thus, the identification of at least one indicator for the early detection of hepatic fibrosis with high degrees of repeatability and reliability is particularly important. The performance of multiple IVIM parameters for hepatic fibrosis detection is unclear and controversial. At present, there are some deficiencies in IVIM and liver fibrosis research, such as only one observer (6,7) or just focus the whole liver lobe(7) rather than the liver segments. Therefore, this study was to evaluate the repeatability and reliability of DWI parameters [apparent diffusion coefficient (ADC) derived from a mono-exponential model; D, D*, and f computed based on a biexponential model, and the DDC and intravoxel heterogeneity index (α) calculated from a stretched exponential model] retrieved from right-lobe segments in patients with early hepatic fibrosis and healthy volunteers. To evaluate the repeatability and reliability of hepatic DWI data from patients with early liver fibrosis and healthy volunteers.Materials and Methods
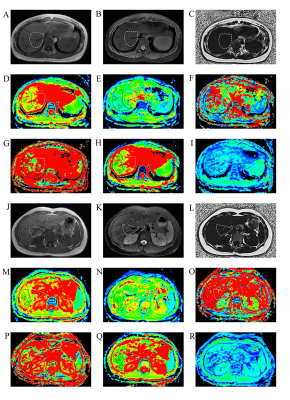
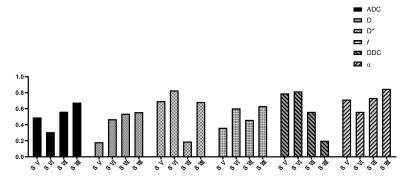
26 patients and 24 healthy volunteers underwent magnetic resonance imaging including respiratory-triggered single-shot spin echo–based echo planar DWI, with 12 b values and an IDEAL-IQ sequence at 3.0 T MR (Signa Discover 750w, GE Healthcare). Two readers independently analyzed image data and drew three ROIs on each segment (V-VIII) on maps of ADC, D, D*, f, DDC, and α(Figure 1). Repeatability and interobserver reliability were assessed using coefficients of variation (CVs) and intraclass correlation coefficients (ICCs) before and after exclusion of subjects with potential liver fat deposition.Results
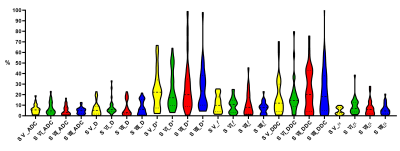
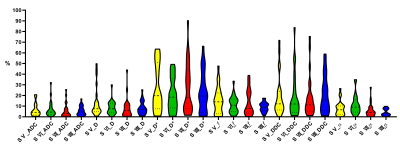
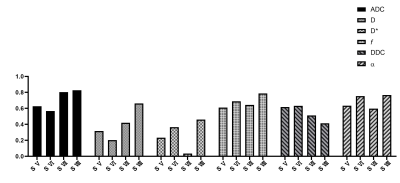
CVs of ADC, D and α were satisfactory for patients (3.29–5.53%, 2.96–6.18%, 4.61–8.35%) and volunteers (3.24–4.51%, 5.77–7.51%, 2.90–6.55%). Except D and DDC, CVs of D*, f , and DDC were poorer; segment-Ⅶ and -Ⅷ values were similar to or better than other segment values (Figure 2 & 3). ICC was similar to patients and volunteers for different parameters in different segments. In particular, ICC was satisfactory to good for segment-VIII ADC (0.827, 0.667) and segment-V DDC (0.617, 0.792) for both patients and healthy volunteers (Figure 4 & 5). The exclusion of subjects with fat deposition did not bring significantly different results.Discussion and Conclusions
The blood flow distribution in the liver is heterogeneous (8,9). To avoid obvious vessels and bile ducts, radiologists typically select the peripheral hepatic parenchyma for IVIM measurements. Peripheral liver tissues distal to the main blood-supplying vessels showed less perfusion but more diffusion, attributing to peripheral liver tissues showing relative homogeneity on ADC and D maps in in this study. Compared with segments Ⅴ and Ⅵ, segments Ⅶ and Ⅷ have larger cross-sections, and measurements of their peripheral tissues were relatively stable. The flow velocity and perfusion of vascular blood as well as tubular fluid on pseudo-colored D* maps but not on conventional T1- and T2-weighted images for reference showed heterogeneous and poor repeatability of measurements. Segments V and VI possess relatively smaller cross-sections and volumes compared to of segments Ⅶ and Ⅷ, resulting in more ROI overlapped. The repeatability of the DDC was similar to that of D* and inferior to that of the ADC, D, f and α, and its reliability was poor in both groups in this study. CVs and ICCs for α were good in this study, but animal and human studies have yielded unclear results regarding the significance of α as a imaging biomarker for liver fibrosis (10). In conclusion, ADC, D and α measurements of right liver lobe were satisfactorily repeatable in early fibrosis and healthy liver tissues, regardless of fat deposition. Segment-VII and -VIII values may be more repeatable and reliable than other segments. Given its poorer repeatability, the explanation and utilities of D* and DDC measurement should be carefully addressed.Acknowledgements
Funding: This project was no funding.References
1. Le Bihan D. What can we see with IVIM MRI? Neuroimage 2019;187:56-67.
2. Szubert-Franczak AE, Naduk-Ostrowska M, Pasicz K, Podgorska J, Skrzynski W, Cieszanowski A. Intravoxel incoherent motion magnetic resonance imaging: basic principles and clinical applications. Pol J Radiol 2020;85:e624-e635.
3. Tosun M, Onal T, Uslu H, Alparslan B, Cetin Akhan S. Intravoxel incoherent motion imaging for diagnosing and staging the liver fibrosis and inflammation. Abdom Radiol (NY) 2020;45(1):15-23.
4. Sandrasegaran K, Territo P, Elkady RM, Lin Y, Gasparis P, Borthakur G, Lin C. Does intravoxel incoherent motion reliably stage hepatic fibrosis, steatosis, and inflammation? Abdom Radiol (NY) 2018;43(3):600-606.
5. Ren H, Liu Y, Lu J, An W, Wang W, Yan T, Li Y, Dong J, Cai J. Evaluating the clinical value of MRI multi-model diffusion-weighted imaging on liver fibrosis in chronic hepatitis B patients. Abdom Radiol (NY) 2021;46(4):1552-1561.
6. Lee Y, Lee SS, Kim N, Kim E, Kim YJ, Yun SC, Kuhn B, Kim IS, Park SH, Kim SY, Lee MG. Intravoxel incoherent motion diffusion-weighted MR imaging of the liver: effect of triggering methods on regional variability and measurement repeatability of quantitative parameters. Radiology 2015;274(2):405-415.
7. Franca M, Marti-Bonmati L, Alberich-Bayarri A, Oliveira P, Guimaraes S, Oliveira J, Amorim J, Gonzalez JS, Vizcaino JR, Miranda HP. Evaluation of fibrosis and inflammation in diffuse liver diseases using intravoxel incoherent motion diffusion-weighted MR imaging. Abdom Radiol (NY) 2017;42(2):468-477.
8. Taniguchi H, Oguro A, Takeuchi K, Miyata K, Takahashi T, Inaba T, Nakahashi H. Difference in regional hepatic blood flow in liver segments--non-invasive measurement of regional hepatic arterial and portal blood flow in human by positron emission tomography with H2(15)O. Ann Nucl Med 1993;7(3):141-145.
9. Van de Wiele C, Maes A, Brugman E, D'Asseler Y, De Spiegeleer B, Mees G, Stellamans K. SIRT of liver metastases: physiological and pathophysiological considerations. Eur J Nucl Med Mol Imaging 2012;39(10):1646-1655.
10. Anderson SW, Barry B, Soto J, Ozonoff A, O'Brien M, Jara H. Characterizing non-gaussian, high b-value diffusion in liver fibrosis: Stretched exponential and diffusional kurtosis modeling. J Magn Reson Imaging 2014;39(4):827-834.
Figures