4653
Tales of Two Environments: Enriched Mice show Stronger Sensory Evoked BOLD fMRI Responses, while Socially Isolated Ones Respond Less
Taekwan Lee1, Taeyi You2,3, Geun Ho Im3, Seong-Gi Kim2,3,4, Sungkwon Chung5, and Jung Hee Lee2,3,6
1Korea Brain Research Institute, Daegu, Korea, Republic of, 2Biomedical Engineering, Sungkyungkwan University, Suwon, Korea, Republic of, 3Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 4Intelligent Precision Healthcare Convergence, Sungkyungkwan University, Suwon, Korea, Republic of, 5Physiology, Sungkyunkwan University, Suwon, Korea, Republic of, 6Radiology, Sungkyungkwan University, Suwon, Korea, Republic of
1Korea Brain Research Institute, Daegu, Korea, Republic of, 2Biomedical Engineering, Sungkyungkwan University, Suwon, Korea, Republic of, 3Center for Neuroscience Imaging Research, Institute for Basic Science, Suwon, Korea, Republic of, 4Intelligent Precision Healthcare Convergence, Sungkyungkwan University, Suwon, Korea, Republic of, 5Physiology, Sungkyunkwan University, Suwon, Korea, Republic of, 6Radiology, Sungkyungkwan University, Suwon, Korea, Republic of
Synopsis
It is well known environmental factors can affect brain plasticity in humans, yet finding strong correlative factors is difficult due to the long development and complexity of human research. Mouse enrichment studies allows for better controlled research and by combining it with fMRI, makes mapping brain-wide plasticity changes possible. Here, we treated mice into three groups of enrichment, standard caging, and isolated caging to see how their brain responds to multiple-sensory stimulations. We found the enrichment group responded stronger in multimodal midbrain and thalamic areas. The isolated group responded less suggesting mouse fMRI is viable in detecting plasticity changes.
Introduction
Functional MRI has become an increasingly popular tool to investigate brain function for translational neuroscience. Studies investigating the functional changes due to disease or functional role of certain nuclei within the brain have been well explored with fMRI in humans and rodents. Various brain imaging studies have shown functional changes in humans, particularly children, who grew up in different environmental conditions. This suggests fMRI is capable in detecting subtle brain plasticity changes caused by environmental factors, yet whether these changes are detectable with rodent fMRI have yet to be shown. Rodent studies utilizing an “enriched” environment have shown cellular, molecular, and behavioral improvements in both wild-type and diseased models1. An “enriched” environment offers novel stimuli that can promote somatosensory, motor, visual, and cognitive plasticity compared to the standard caging mice are kept. As environmental modulation is a potential therapeutic treatment option for many brain disorders, obtaining in-vivo brain-wide functional changes with mice fMRI can help supplement its progress and understanding on brain plasticity. To investigate if such changes are observable, we utilized a multi-sensory stimulation approach where we attempt to see whisker-pad, forepaw, visual, and olfactory response in mice that have been exposed to an enriched environment, standard, or isolated cagings.Methods
Thirty-two C57BL/6 mice were used to investigate the effects of the housing environment via fMRI. Twelve mice (in groups of 4) were housed in an “enriched” environment (EE) in which mice had free access to a 30cm long tunnel, an igloo nest and two running wheels in an enlarged 50x50cm space. Twelve mice (in groups of 4) were kept in standard laboratory cages (SC) of 30x22cm. Eight mice were isolated into individual mice cages (IC) of 10x30cm. All mice were put into their respective environments post-weaning at 4-weeks of age and kept there for 6 weeks before fMRI experimentation. Multi-sensory stimulation fMRI experiments were conducted at 15.2T/11cm Bruker Biospec System under continuous i.v. ketamine/xylazine anesthesia described previously2. Left forepaw (FP) was electrically stimulated subcutaneously with 0.6mA at 4Hz, and right whisker-pad (WP) was electrically stimulated with 0.4mA at 4Hz. Binocular visual stimulation (VIS) was conducted with white LED lights at 4Hz. Olfactory stimulation (OLF) was conducted with 5% amyl acetate mixed in mineral oil delivered through the O2/air gas mixture. Mice were stimulated in block design of 40s baseline, 20s stimulation, 60s interstimulus interval, 20s stimulation, and 60s recovery. Stimulation types were randomly done until BOLD response in the primary cortical area of each respective modality was acquired. Images were preprocessed and analyzed via GLM. BOLD amplitude from area-under-curve (AUC) was calculated by averaging the area of the two response peaks.Results
All stimulation modalities resulted in expected activation in the respective sensory axis2,3. OLF stimulation resulted in activation in the main olfactory bulb (MOB) and piriform cortex (PIR) along with downstream regions such as prefrontal cortex, medial dorsal thalamus (MD), and hippocampal formation (Figure 1). Olfactory stimulation did not have any significant differences among EE, SC, and IC groups. With WP stimulation, significantly higher response in the midbrain reticular nucleus (MRN) and substantia nigra pars reticulata (SNr) in the midbrain, and ventral posterior (VP), posterior (POm), subparafasicular area (SPA), and ventral anterolateral (VAL) in the thalamus were observed in EE compared to SC and IC (Figure 2). FP stimulation resulted in significantly higher response in the ventral retrosplenial cortex (RSCv) of the EE group (Figure 3). VIS stimulation resulted in significantly lower activation in the IC group in lateral dorsal (LD) and lateral posterior (LP) thalamus, posterior parietal cortex (PPCa), and primary and secondary visual cortices compared to EE and SC (Figure 4).Discussion
EE group presented with higher BOLD activity in midbrain (MRN, SNr) and thalamic (VP, POm, VAL, SPA) areas from WP stimulation compared to SC and IC groups. MRN contains high connectivity to the thalamus and cortex and modulates ascending sensory signals. SNr is involved in motor execution. The enhanced thalamic responses were found to be involved in cortico-thalamo-cortical modulation of the sensorimotor pathway4. Similarly, FP stimulation resulted in higher activity in the RSCv in the EE group which is known to be a hub for integrating sensorimotor signals5. The enhanced activity in EE suggests functional plasticity changes within the sensorimotor pathway due to the increased movement and exploration. Contrarily, IC group presented with lower BOLD activity from VIS stimulation within the visual cortex (V1/V2) and thalamus (LD/LP), along with reduced activity in the PPCa which is involved in coordinating action from multimodal sensory inputs. This may be due to the lack of visual and sensory input for the IC group which may have resulted in reduced function in these cortical areas. The lack of difference from OLF stimulation may be due to not providing novel olfactory stimuli for our EE group.Conclusion
We have shown the feasibility in detecting functional plasticity changes brought upon by environmental conditions in which mice are housed with fMRI. We see a possible gain-of-function in midbrain and thalamic nuclei in the EE group related to sensorimotor pathways. A loss-of-function was observed in the IC group in response to VIS stimulation. These nuclei may be potential targets for future studies regarding enrichment plasticity.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (NRF-2020R1A2C2012416).References
- Nithianantharajah, J., Hannan, A. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci 7, 697–709 (2006).
- You, T., Im, G.H. & Kim, SG. Characterization of brain-wide somatosensory BOLD fMRI in mice under dexmedetomidine/isoflurane and ketamine/xylazine. Sci Rep 11, 13110 (2021).
- Dinh TNA, Jung WB, Shim HJ, Kim SG. Characteristics of fMRI responses to visual stimulation in anesthetized vs. awake mice. Neuroimage. 2021 Feb 1;226:117542.
- Mo, Christina, and S. Murray Sherman. “A Sensorimotor Pathway via Higher-Order Thalamus.” The Journal of Neuroscience, vol. 39, no. 4, 2018, pp. 692–704.
- Mao, D., Molina, L. A., Bonin, V. & McNaughton, B. L. Vision and Locomotion Combine to Drive Path Integration Sequences in Mouse Retrosplenial Cortex. Curr. Biol. 30, 1680-1688.e4 (2020).
Figures
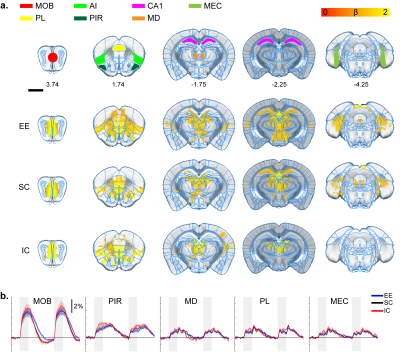
Olfactory stimulation response map. A.
ROI definition of related sensory regions and response map of EE, SC, and IC
groups. MOB, main olfactory bulb; PL, prelimbic; AI, agranular insula; PIR,
piriform cortex; CA1, hippocampal CA1 subfield; MD, mediodorsal thalamus; MEC,
medial entorhinal cortex. P=0.05 corrected B. Percent signal change. Gray bar
represents stimulation time
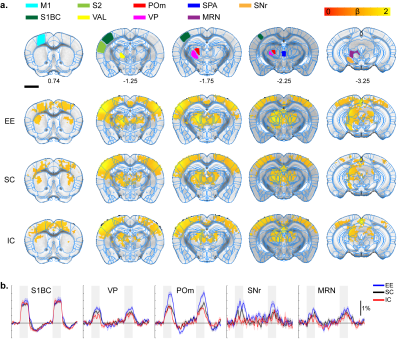
Whisker-pad stimulation response map. A.
ROI definition of related sensory regions and response map of EE, SC, and IC
groups. M1, primary motor cortex; S1BC, primary somatosensory barrel cortex;
S2, secondary somatosensory cortex; VAL, ventral anterolateral thalamus; POm,
posterior thalamus; VP, ventral posterior thalamus; SPA, subparafasicular
thalamus; MRN, midbrain reticular nucleus; SNr, substantia nigra. P=0.05
corrected B. Percent signal change. Gray bar represents stimulation time
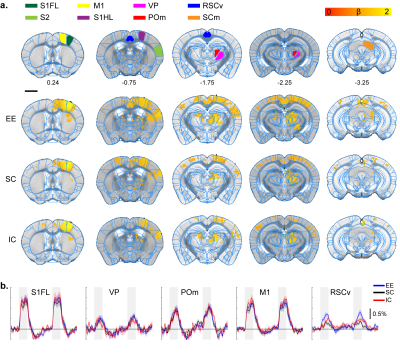
Forepaw stimulation response map.
A. ROI definition of related sensory regions
and response map of EE, SC, and IC groups. S1FL, primary somatosensory
forelimb; S1HL, primary somatosensory hindlimb; RSCv, ventral retrosplenial
cortex; SCm, superior colliculus motor.
P=0.05 corrected B. Percent signal change.
Gray bar represents stimulation time
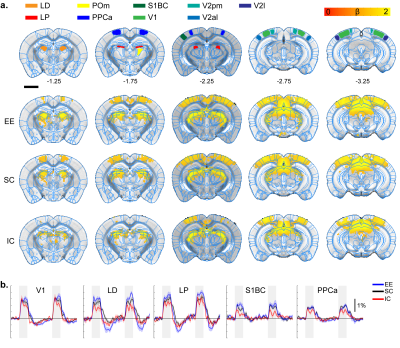
Visual stimulation response map.
A. ROI definition of related sensory
regions and response map of EE, SC, and IC groups. LD, lateral dorsal thalamus;
LP, lateral posterior thalamus; PPCa, posterior parietal cortex; V1, primary
visual cortex; V2pm/al/l, secondary visual posteromedial/anterolateral/lateral.
P=0.05 corrected B. Percent signal change.
Gray bar represents stimulation time
DOI: https://doi.org/10.58530/2022/4653