4651
Detection of early metastatic lymph nodes using inflow-based vascular-space-occupancy (iVASO) MR imaging1Department of Medical Imaging, Nanfang Hospital, Southern Medical University, Guangzhou, China, 2Neurosection, Division of MRI Research, Department of Radiology,, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 3F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4Philips Healthcare, Guangzhou, China
Synopsis
Accurate preoperative evaluation of lymph nodes (LNs) involvement is of vital importance for clinical decisions and optimizing individual treatment regimens. Related studies showed elevated perfusion at the level of capillary in metastatic LNs. Inflow-based vascular space occupancy (iVASO) is a novel and noninvasive perfusion technology that can provide absolute blood volume of precapillary arterioles (arteriolar blood volume, BVa). In this study, the potential value of BVa in detecting metastatic LNs was investigated. The results showed that the BVa might have the potential to detect early metastases within lymph nodes.
Purpose
Currently, morphological criteria such as size and shape are commonly used for the characterization of nodal involvement clinically. However, the main drawbacks of size-based metrics for detecting metastases are the variable criterion and the inconsistent sensitivity across different studies [1, 2]. Previous studies showed the angiogenesis in the process of nodal invasion by tumor, leading to hyperperfusion of metastatic LNs [3]. Perfusion weighted imaging can provide quantitative information on the microcirculation of LNs, and thus reflect the nature of the disease more accurately. Inflow-based vascular-space-occupancy (iVASO) is a novel perfusion technique without the need for exogenous contrast agents [4]. This study aimed to investigate the potential value of iVASO MR imaging in differentiating metastatic from inflammatory lymph nodes (LNs).Methods
Ten female New Zealand rabbits with 2.5-3.0 kg body weight were studied. VX2 cells and egg yolk emulsion were inoculated into left and right thighs, respectively, to induce ten metastatic and ten inflammatory popliteal LNs. Conventional MRI and iVASO were performed 2 h prior to, and 10, 20 days after inoculation (D0, D10, D20) with a 3T clinical scanner (Achieva TX, Philips). The short-axis diameter (S), short- to long-axis diameter ratio (SLR), and arteriolar blood volume (BVa) at each time point and their longitudinal changes of each model were recorded and compared. At D20, all rabbits were sacrificed to perform histological evaluation after the MR scan.Results
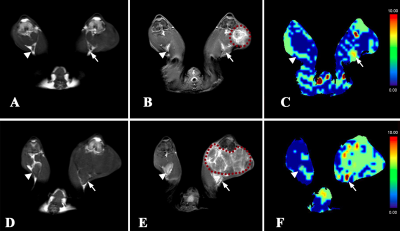
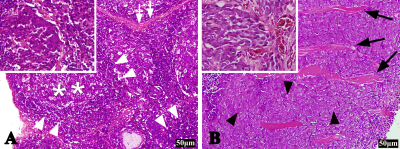
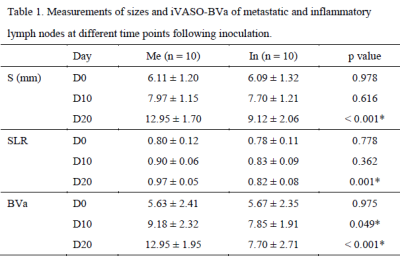
The mean values of S, SLR and BVa showed no significant difference between the two groups at D0 (P = 0.987, P = 0.778, P = 0.975). The BVa of the metastatic group was higher than that of the inflammatory at both D10 and D20 (P < 0.05; P < 0.001), whereas the S and SLR of the metastatic group were greater only at D20 (P < 0.001; P = 0.001) (Table 1). Longitudinal analyses showed that the BVa of the metastatic group increased at both D10 and D20 (P = 0.004; P = 0.001), while that of the inflammatory group only increased at D10 (P = 0.024) (Figure 1). Figure 2 displays the conventional images and BVa maps of the two groups of LNs at D10 and D20. The metastatic LNs showed obvious vasodilation and neovascularization compared with inflammatory LNs (Figure 3).Discussion
The present study showed that the BVa value measured with iVASO changed substantially in both metastatic and inflammatory LNs. Significant differences in the BVa value existed between the two groups at both D10 and D20, whereas the significant difference of size existed only at D20.Morphological criteria, such as size and shape, are commonly used for the characterization of nodal involvement clinically. However, in the present study, the size-based criteria cannot sensitively detect nodal involvement in the early period of metastasis. PWI can provide quantitative information on the microcirculation of LNs, and thus reflect the nature of the disease more accurately. Many previous studies have shown the angiogenesis in the process of nodal invasion by tumor, leading to hyperperfusion of metastatic LNs [5, 6]. In the present study, the popliteal nodes showed a significant and continuing increase of BVa after inoculation of tumor cells into the posterior thigh muscle, indicating a gradually developing process of nodal invasion during which angiogenesis gradually increased within the nodes [3]. In contrast, the BVa value of the inflammatory LNs did not show a continual increase. This was likely because the vasodilation subsided with the resolution of inflammation [7, 8]. It is worth noting that previous studies investigated the microcirculation at the capillary level, whereas iVASO predominantly reflects the arteriolar compartment, which is the most sensitive part in response to metabolic disturbance [9, 10]. However, our study did not compare iVASO with DCE-MRI or IVIM-DWI in this regard, so whether iVASO is more sensitive in detecting metastatic LNs than other perfusion technologies warrants further investigation.
Conclusion
In conclusion, this preliminary experiment demonstrated that the arteriolar blood volume measured with iVASO can reflect the perfusion changes of both metastatic and inflammatory lymph nodes, and the BVa might have the potential to detect early metastases within lymph nodes.Acknowledgements
No acknowledgement found.References
[1] Grone J, Loch FN, Taupitz M, Schmidt C, Kreis ME. Accuracy of various lymph node staging criteria in rectal cancer with magnetic resonance imaging. J Gastrointest Surg 2018; 22:146-153.
[2] Liu Z, Feng B, Li C, et al. Preoperative prediction of lymphovascular invasion in invasive breast cancer with dynamic contrast-enhanced-MRI-based radiomics. J Magn Reson Imaging 2019; 50:847-857.
[3] Guo L, Liu X, Liu Z, et al. Differential detection of metastatic and inflammatory lymph nodes using intravoxel incoherent motion diffusion-weighted imaging. Magn Reson Imaging 2020; 65:62-66.
[4] Hua J, Qin Q, Pekar JJ, et al. Measurement of absolute arterial cerebral blood volume in human brain without using a contrast agent. NMR BioMed. 2011; 24:1313-1325
[5] Zhu Y, Li X, Wang F, et al. Intravoxel incoherent motion diffusion-weighted magnetic resonance imaging in characterization of axillary lymph nodes: Preliminary animal experience. Magn Reson Imaging 2018; 52:46-52.
[6] Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjosne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000; 10:1464-1471.
[7] RH Farnsworth MLMA. Vascular remodeling in cancer. Oncogene 2013:3496-3505.
[8] Soderberg KA, Payne GW, Sato A, Medzhitov R, Segal SS, Iwasaki A. Innate control of adaptive immunity via remodeling of lymph node feed arteriole. Proc Natl Acad Sci U S A 2005; 102:16315-16320.
[9] Wu Y, Agarwal S, Jones CK, et al. Measurement of arteriolar blood volume in brain tumors using MRI without exogenous contrast agent administration at 7T. J Magn Reson Imaging 2016; 44:1244-1255.
[10] Li X, Wang D, Liao S, et al. Discrimination between glioblastoma and solitary brain metastasis: comparison of inflow-based vascular-space-occupancy and dynamic susceptibility contrast MR imaging. AJNR Am J Neuroradiol 2020; 41:583-590.
Figures