4647
Glycolytic Flux Alterations Following Radiotherapy in Cancer and Immune Cells: Hyperpolarized Carbon-13 Magnetic Resonance Imaging Study
Ying-Chieh Lai1,2, Ching-Yi Hsieh2,3,4, Kuan-Ying Lu1,2, Albert P Chen5, Shu-Hang Ng1,4, Fang-Hsin Chen4,6, and Gigin Lin1,2,3,4
1Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital at Linkou and Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 2Clinical Metabolomics Core Laboratory, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Taoyuan, Taiwan, 3Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 4Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 5GE Healthcare, Toronto, ON, Canada, Toronto, ON, Canada, 6Radiation Biology Research Center, Institute for Radiological Research, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan
1Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital at Linkou and Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 2Clinical Metabolomics Core Laboratory, Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan, Taoyuan, Taiwan, 3Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 4Department of Medical Imaging and Radiological Sciences, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan, 5GE Healthcare, Toronto, ON, Canada, Toronto, ON, Canada, 6Radiation Biology Research Center, Institute for Radiological Research, Chang Gung University, Taoyuan, Taiwan, Taoyuan, Taiwan
Synopsis
Alterations in metabolism following radiotherapy affect therapeutic efficacy, although the mechanism underlying such alterations is unclear. The present study demonstrates the potential for monitoring early metabolic alterations in both cancer and immune cells following radiation treatment by using hyperpolarized 13C-MRI. We demonstrated that cancer cells (human FaDu squamous carcinoma) and immune cells (HMC3 microglial cells and THP-1 monocytes) had distinct metabolic responses to ionizing radiation. Western blot analysis confirmed the similar trends in LDHA and LDHB expression levels. We furthered the knowledge of metabolic alterations resulting from not only cancer cells but also immune cells in the tumor microenvironment.
Introduction
Radiotherapy is an important treatment for various cancer types. The metabolic reprogramming of cancer and stromal cells in the tumor microenvironment is an important mechanism of radioresistance [1–3]. The enhanced cellular utility of glucose in cancer cells, termed the Warburg effect, has been associated with radioresistance [4–6]. Pyruvate, the product of glycolysis, is reduced to lactate by lactate dehydrogenase (LDH), while lactate is further used as a metabolic shuttle to generate more energy (Figure 1) [7]. Immune cells in the tumor microenvironment may also undergo substantial metabolic reprogramming and contribute to radioresistance [8,9]. We hypothesized that in response to irradiation, cancer and immune cells may have different alterations in glycolytic flux. To understand the potential contributions of the overall glycolytic activities in tumors in response to irradiation, we designed a DNP 13C-MRI study by using [1-13C]pyruvate to probe cancer and immune cell lines.Methods
[1-13C]pyruvate were polarized using a clinical hyperpolarizer (SPINlab). Human FaDu squamous carcinoma from hypopharyngeal cancer was selected for the cancer cell model. HMC3 microglial cells (specialized macrophages in the brain) were used to represent the tissue-resident immune cells, while THP-1 monocytes were used to represent the immune cells recruited from peripheral blood cells. The three cell lines were split into samples that were or were not subjected to 15 Gy X-ray irradiation. After 30–79 min (mean, 53 min) following irradiation, the samples were imaged by using clinical 3T MRI system (Discovery MR750w).Results
The intensities of the labeled pyruvate and lactate from 13C-MR spectra were fit to a two-site exchange model [10]. Representative 13C-MR spectra and images of the cell experiments are presented in Figure 2. The data for the pyruvate-to-lactate conversion rate (kPL [Pyr.]) obtained through kinetic modeling are summarized in Figure 2. The irradiated FaDu cancer cells showed a significant decrease in the pyruvate-to-lactate conversion rate from 38.1 ± 3.6 to 22.9 ± 11.6 nM/s/106 cells (mean ± standard deviation, p = 0.049). The irradiated HMC3 microglial cells also showed a significant decrease in the pyruvate-to-lactate conversion rate from 25.7 ± 3.4 to 16.4 ± 2.2 nM/s/106 cells (p = 0.008). In contrast, the irradiated THP-1 monocytes showed an increase in the pyruvate-to-lactate conversion rate from 17.9 ± 11.1 to 24.6 ± 5.2 nM/s/106 cells, albeit not statistically significant (p = 0.199). Moreover, [1-13C]alanine was observed in both irradiated and non-irradiated HMC3 microglial cell experiments. Since the conversions between hyperpolarized [1-13C]pyruvate and [1-13C]lactate are mediated by LDHA (forward reaction) and LDHB (backward reaction), Western blot was performed to evaluate the expression of these key metabolic proteins before and after irradiation (Figure 3). The irradiated FaDu cancer cells showed significantly decreased expression levels of LDHA and LDHB (p = 0.049 and 0.001, respectively). The irradiated HMC3 microglial cells also showed significantly decreased expression levels of LDHA and LDHB (p = 0.002 and 0.014, respectively). In contrast, the irradiated THP-1 monocytes showed increased expression levels of LDHA and LDHB, albeit not statistically significant (p = 0.092 and 0.107, respectively). Compared to the results from DNP 13C-MRI, Western blot analysis confirmed the similar trends in LDHA and LDHB expression levels.Discussion
The present study demonstrates the potential for monitoring early metabolic alterations in both cancer and immune cells following radiation treatment by using hyperpolarized 13C-MRI. We demonstrated that cancer cells (human FaDu squamous carcinoma) and immune cells (HMC3 microglial cells and THP-1 monocytes) had distinct metabolic responses to ionizing radiation. MR spectroscopy with a 13C-labeled substrate was applied to study cancer cell metabolism in preclinical settings [11,12]. We furthered the knowledge of metabolic alterations resulting from not only cancer cells but also immune cells in the tumor microenvironment [13].Acknowledgements
This research was funded by BMRPF63, the Chang Gung Foundation; CLRPG3K0021, the Chang Gung Foundation; CMRPD1J0322, the Chang Gung Foundation; CMRPD1H0473, the Chang Gung Foundation; MOST107-2221-E-182-023, the Ministry of Science and Technology Taiwan; MOST109-2628-B-182A-007, the Ministry of Science and Technology Taiwan; MOST-109-2628-B-182-008, the Ministry of Science and Technology Taiwan; MOST109-2314-B-182-078-MY3, the Ministry of Science and Technology Taiwan; MOST107-2314-B-182A-059-MY2, the Ministry of Science and Technology Taiwan; NHRI-109BCCO-MF-202009-02, the National Health Research Institutes Taiwan.References
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 1–15.
- Lin, J.; Xia, L.; Liang, J.; Han, Y.; Wang, H.; Oyang, L.; Tan, S.; Tian, Y.; Rao, S.; Chen, X.; et al. The roles of glucose metabolic reprogramming in chemo- and radio-resistance. J. Exp. Clin. Cancer Res. 2019, 38, 1–13.
- Cruz-Gregorio, A.; Martinez-Ramirez, I.; Pedraza-Chaverri, J.; Lizano, M. Reprogramming of energy metabolism in response to radiotherapy in head and neck squamous cell carcinoma. Cancers 2019, 11, 182.
- Zhao, F.; Ming, J.; Zhou, Y.; Fan, L. Inhibition of Glut1 by WZB117 sensitizes radioresistant breast cancer cells to irradiation. Cancer Chemother. Pharmacol. 2016, 77, 963–972.
- Koukourakis, M.I.; Giatromanolaki, A.; Winter, S.; Leek, R.; Sivridis, E.; Harris A.L. Lactate dehydrogenase 5 expression in squamous cell head and neck cancer relates to prognosis following radical or postoperative radiotherapy. Oncology 2009, 77, 285–292.
- Quennet, V.; Yaromina, A.; Zips, D.; Rosner, A.; Walenta, S.; Baumann, M.; Mueller-Klieser, E. Tumor lactate content predicts for response to fractionated irradiation of human squamous cell carcinomas in nude mice. Radiother Oncol. 2006, 81, 130–135.
- Draoui, N.; Feron, O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis. Model. Mech. 2011, 4, 727–732.
- Biswas, S.K. Metabolic reprogramming of immune cells in cancer progression. Immunity 2015, 43, 435–449.
- Kumagai, K.; Akakabe, M.; Tsuda, M.; Tsuda, M.; Fukushi, E.; Kawabata, J.; Abe, T.; Ichikawa, K. Observation of glycolytic metabolites in tumor cell lysate by using hyperpolarization of deuterated glucose. Biol. Pharm. Bull. 2014, 37, 1416–1421.
- Harrison, C.; Yang, C.; Jindal, A.; DeBerardinis, R.J.; Hooshyar, M.A.; Merritt, M.; Sherry, A.D.; Malloy, C.R. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13C NMR. NMR Biomed. 2012, 25, 1286–1294.
Figures
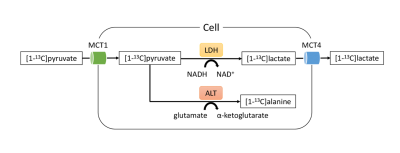
Diagram of the metabolic fates of [1-13C]pyruvate that were detected by hyperpolarized 13C-MR spectroscopy in this study. Note—MCT, monocarboxylate transporter; LDH, lactate dehydrogenase; ALT, alanine transaminase.
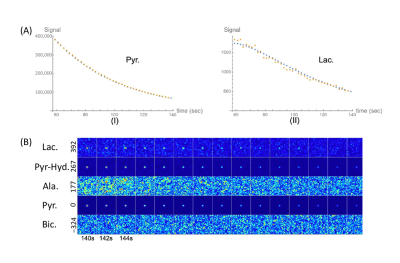
Representative data from an in vitro study. (A) Orange dots represent the data for [1-13C]pyruvate (I) and [1-13C]lactate (II) obtained from the spectroscopy-based 13C-MRI. Blue dots represent best fit lines calculated by kinetic modeling. (B) Imaging-based 13C-MRI acquisition of the investigated metabolites displayed at a temporal resolution of 2 s. Each row represents one metabolite: lactate (392 Hz), pyruvate–hydrate (267 Hz), alanine (177 Hz), pyruvate (0 Hz), and bicarbonate (−324 Hz). 13C signals of lactate, pyruvate–hydrate, and pyruvate were visually observed.
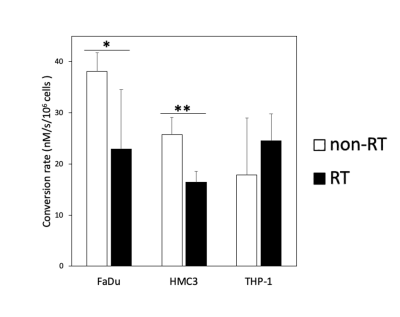
Comparison of the conversion rates (kPL [Pyr.]) of hyperpolarized [1-13C]pyruvate to [1-13C]lactate between the non-irradiated (non-RT) and irradiated (RT) FaDu, HMC3, and THP-1 cells (* p < 0.05; ** p < 0.01). Note—kPL [Pyr.] unit: nM/s/106 cells.
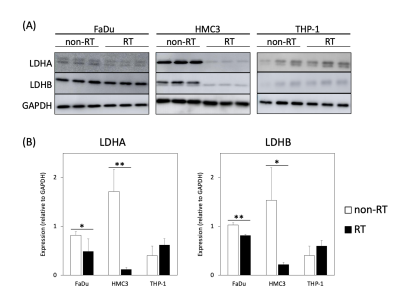
Analysis of the expressions of LDHA and LDHB in the non-irradiated (non-RT) and irradiated (RT) cancer and immune cells. (A) Western blot analyses of LDHA and LDHB in FaDu, HMC3, and THP-1 cells. GAPDH was used as a loading control. (B) Comparison of the expressions of LDHA and LDHB between the non-irradiated and irradiated cells (* p < 0.05; ** p < 0.01). Note—LDH, lactate dehydrogenase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
DOI: https://doi.org/10.58530/2022/4647