4645
Design and construction of a volume Tx coil and conformal Rx array for simultaneous squirrel monkey brain and spinal cord imaging at 9.4 T
Ming Lu1, Zhangyan Yang2,3, Feng Wang2,4, Gary Drake2,4, Li Min Chen2,4, John Gore2,3,4, and Xinqiang Yan2,4
1College of nuclear equipment and nuclear engineering, Yantai University, Yantai, China, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 3Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
1College of nuclear equipment and nuclear engineering, Yantai University, Yantai, China, 2Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 3Department of Biomedical Engineering, Vanderbilt University, Nashville, TN, United States, 4Department of Radiology and Radiological Sciences, Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
Simultaneous imaging of brain and spinal cord regions potentially provides valuable information about how they work together and interact. However, to date, almost all studies have investigated these two highly interconnected systems separately, mainly because of a lack of adequate imaging coils. We designed a volume Tx and conformal receive array dedicated to simultaneous imaging of squirrel monkey brain and spinal cord at 9.4 T. The coil exhibits excellent SNR in both brain and spinal cord areas and can be used for studies investigating how the brain and spinal cord work together.
Introduction:
Functional and structural MRI play important roles in understanding the relationships between the organization of spinal cord and brain [1-3]. For example, the processing of innocuous touch and painful stimuli engages circuits within the grey matters of both spinal cord and the brain’s somatosensory cortex [4]. These spinal cord and brain regions interact constantly to execute behavioral demands originating in the brain’s high cognitive areas such as goal-oriented limb movement. Simultaneous imaging of these separate regions potentially provides valuable information about how they work together and interact. However, to date, almost all studies have investigated these two highly interconnected systems separately, mainly because of a lack of adequate imaging coils. Simultaneous brain and spinal cord imaging requires a volume coil for transmit and local multiple coil arrays for reception in order to achieve uniform transmit fields as well as optimal signal-to-noise ratio in both brain and spinal cord regions. Although dedicated Rx arrays for brain and C-spine have been developed for human scanners [5-6], such a coil for NHP imaging in preclinical scanners has not previously been described. The target of this work was therefore to design and construct a Tx/Rx array for simultaneous brain and spinal cord imaging of squirrel monkeys.Methods:
Overall systemThe coil (volume Tx + 4-ch receive array) and animal holding system were designed and constructed for a Varian DirectDriveTM horizontal 9.4-T preclinical scanner (bore clearance 11.7 cm) which provides one Tx and 4 Rx channels for proton imaging. Figure 1 depicts how the volume Tx coil and receive array were interfaced to the scanner.
Volume Tx coil
The Tx coil is a high-pass quadrature birdcage with up-to-16 legs and thereby able to provide a homogenous area over a large volume (Figure 2a). The volume coil has a length of 10 cm and an inner diameter of 8.5 cm. Cable outer conductors were directly connected to the RF shield by 330-pF DC-block capacitors to realize a balun-free drive. Due to the proximity of the birdcage coil to the receive array, up to 16 series PIN diodes were employed to achieve detuning in the receive phase. These diodes were connected in series and driven by a single 12V/50mA detune signal. Half of the distributed capacitors (8.2 pF) in the end rings were bridged by RF chokes to allow the DC current to run through all PIN diodes.
Receive array
Similar to state-of-art human coils, the Rx array was arranged to maximize the filling factor and SNR (Figure 2b). The number of Rx coils was chosen to match the available Rx channels. The Rx array consists of 1 top loop covering the anterior brain, one bottom loop covering the posterior brain and the cervical spinal cord, and two loops covering side regions. The coil housing was designed in SolidWorks to match the curves of a squirrel monkey’s head and neck. Adjacent loops were decoupled by overlapping and opposite loops were decoupled with transformers. Shielded cable traps were employed immediately after the Rx coils to reduce the common-mode current flowing on the cable's outer conductor.
Bench test and MR imaging experiment
Prior to MR experiments, properties of both the Tx coil and the receive array were measured on the bench with a four-port Keysight 5071C VNA. We also evaluated the detuning performance of the Tx and Rx coils with a pair of well-decoupled pick-up probes. GRE images using the volume Tx+4-ch Rx array were acquired on a monkey-head-shaped phantom for SNR assessments. As a comparison, we also acquired GRE images on a commercial volume coil from Doty Scientific (inner diameter 8.5 cm).
Results:
Bench testFigure 3 shows the measured S-parameter matrix of quadrature channels of the Tx coil and the four Rx coils. The Rx coil was detuned when measuring the S-parameter of Tx coils, and vice versa. The quadrature ports of the Tx volume coil have high isolation of ~-20 dB, while the inter-element isolation of the receive array ranges from -15.2 dB to -20 dB. Both the transmit coil and the four receive coils could be well-tuned to 400.6 MHz and matched to 50 Ω (S11<-25 dB) with a large variety of loadings by using the tuning rods. Table 1 lists the detune performance of Tx and Rx coils. Excellent detuning performance (<-25 dB) can be achieved by active detune circuits in the Tx coil’s rungs and Rx coils.
SNR maps
Figure 4 plots the multi-slice sagittal SNR maps on the phantom using the Doty coil and the constructed Tx coil+Rx array. Substantial SNR increases were observed by using the close-fitting Rx array, with average SNR gain at brain/c-spinal cord area of ~37%/43%.
Conclusions:
We designed a volume Tx and conformal Rx array dedicated to simultaneous imaging of squirrel monkey brain and spinal cord at 9.4 T. The coil exhibits excellent SNR in both brain and spinal cord areas and can be used for studies investigating how the brain and spinal cord work together.Acknowledgements
This work was supported by NIH grant NS092961 and DOD grant W81XWH-17-1-0304. This work was performed during the period of Dr. Ming Lu’s visit to Vanderbilt University Institute of Imaging Science.References
- P. Nash et al., “Functional magnetic resonance imaging identifies somatotopic organization of nociception in the human spinal cord,” PAIN®, vol. 154, no. 6, pp. 776–781, Jun. 2013, doi: 10.1016/j.pain.2012.11.008.
- J. W. Thorpe, I. F. Moseley, C. H. Hawkes, D. G. MacManus, W. I. McDonald, and D. H. Miller, “Brain and spinal cord MRI in motor neuron disease.,” Journal of Neurology, Neurosurgery & Psychiatry, vol. 61, no. 3, pp. 314–317, Sep. 1996, doi: 10.1136/jnnp.61.3.314.
- R. L. Barry, S. A. Smith, A. N. Dula, and J. C. Gore, “Resting state functional connectivity in the human spinal cord,” eLife, vol. 3, p. e02812, Aug. 2014, doi: 10.7554/eLife.02812.
- “Injury alters intrinsic functional connectivity within the primate spinal cord | PNAS.” https://www.pnas.org/content/112/19/5991.long (accessed Nov. 03, 2021).
- J. Cohen-Adad, A. Mareyam, B. Keil, J. R. Polimeni, and L. L. Wald, “32-Channel RF coil optimized for brain and cervical spinal cord at 3 T,” Magnetic Resonance in Medicine, vol. 66, no. 4, pp. 1198–1208, 2011, doi: 10.1002/mrm.22906.
- B. Zhang, A. C. Seifert, J. Kim, J. Borrello, and J. Xu, “7 Tesla 22-channel wrap-around coil array for cervical spinal cord and brainstem imaging,” Magnetic Resonance in Medicine, vol. 78, no. 4, pp. 1623–1634, 2017, doi: 10.1002/mrm.26538.
Figures
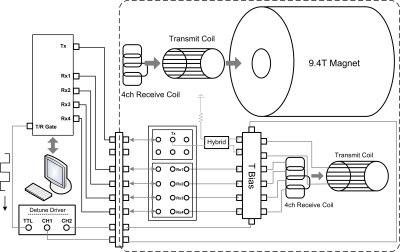
Figure 1 Illustration of
interfacing a volume Tx coil and a 4-channel Rx array to an Agilent/Varian
console.
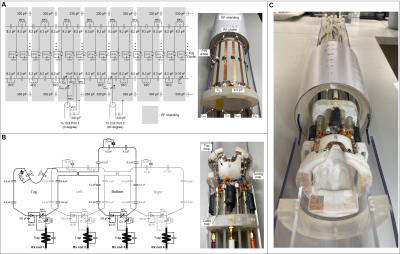
Figure 2 Circuit diagrams and photographs
of the volume Tx coil and the Rx array.
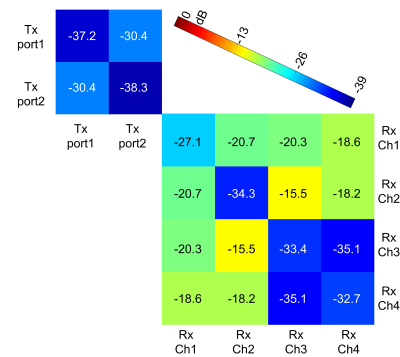
Figure 3 Measured S-parameter
matrix of the volume Tx coil and the Rx array.
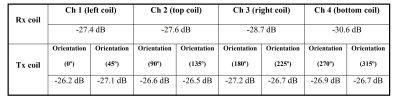
Table
1 Measured detuning performance of the Tx coil and Rx array. Since the Tx coil
is a volume resonator, its complete detuning performance was measured with
double-probes orientated in different directions.

Figure
4 Measured sagittal SNR maps on a monkey-head-shaped phantom using the Doty
85-mm volume coil (top row) and the constructed volume Tx + 4-ch Rx array. SNR
was calculated on low-flip-angle GRE images with the following parameters: FOV
= 85 x 85 mm2, matrix = 256 x 256, TR/TE = 300/4.08 ms, FA = 10
degrees, slice thickness = 1.8 mm, number of averages=2.
DOI: https://doi.org/10.58530/2022/4645