4644
Brain redox imaging using the blood–brain barrier-permeable nitroxides with different resistances to esterase
Miho C EMOTO1, Shingo Sato2, Hideo Sato-Akaba3, and Hirotada G Fujii4
1Department of Clinical Laboratory Science, Health Sciences University of Hokkaido, Sapporo, Japan, 2Yamagata University, Yamagata, Japan, 3Osaka University, Toyonaka, Japan, 4Health Sciences University of Hokkaido, Ishikari, Japan
1Department of Clinical Laboratory Science, Health Sciences University of Hokkaido, Sapporo, Japan, 2Yamagata University, Yamagata, Japan, 3Osaka University, Toyonaka, Japan, 4Health Sciences University of Hokkaido, Ishikari, Japan
Synopsis
Lipophilic 3-methoxycarbonyl-2,2,5,5-tetramethylpiperidine-1-oxyl (MCP) has been used as a redox-sensitive nitroxide probe in MRI and EPR brain redox studies. Despite its success for this purpose, MCP cannot evaluate the redox status at both hydrophobic and hydrophilic brain regions. Accordingly, it is necessary to identify a lipophilic nitroxide that can penetrate the BBB and is hydrolyzed to hydrophilic nitroxide by neuronal esterase in the brain. For this purpose, lipophilic 3-acetoxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl was prepared and compared with MCP. Brain redox EPR imaging revealed that these two probes each detect a different redox status according to the antioxidant levels in the brain.
INTRODUCTION
Under physiological conditions, cells maintain redox balance through generation and elimination of reactive oxygen species. However, disruption of the balance may induce numerous brain disorders such as Alzheimer’s disease. Non-invasive detection of imbalanced redox status is therefore useful for early diagnosis of brain disorders and monitoring their conditions. Nitroxides are relatively non-toxic free radicals and can participate cellular redox reactions, and thus these probes have been widely used as redox-sensitive imaging probes in redox research by magnetic resonance-based imaging modalities. Lipophilic nitroxides have been used as imaging probes to assess brain redox status because of their ability to penetrate the blood-brain barrier (BBB). 3-methoxycarbonyl-2,2,5,5-tetramethylpiperidine-1-oxyl (MCP) has been used as a redox-sensitive probe in redox studies and successfully employed to assess brain redox status in animal disease models. Despite its usefulness, it is doubtful that MCP can evaluate redox status in both hydrophobic and hydrophilic brain regions. To address this question, we examined a different lipophilic nitroxide that can penetrate the BBB, after which it becomes a hydrophilic nitroxide. For this purpose, 3-acetoxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (AMCP) was prepared by masking the carboxyl group of hydrophilic 3-carboxy-2,2,5,5-tetramethylpyrrolidine-1-oxyl (COP) as an esterase-labile ester. We performed electron paramagnetic resonance (EPR) imaging to examine the distribution and reduction kinetics of MCP and AMCP in mouse brains and compared their redox sensitivities.METHODS
Chemicals: MCP was obtained from NARD Chemicals, Ltd. (Osaka, Japan). AMCP was synthesized according to the previously reported method1. The purity of AMCP was determined by EPR and 1H-NMR. Animals: Male C57BL/6 mice aged 6 to 10 weeks with body weights of 20–25 g were used. Mice were anesthetized with 1.5% isoflurane in air at 200 mL/min, and the tail vein was cannulated with a 27-gauge needle for the injection of nitroxides. A solution of both nitroxides (0.75 μmol/g body weight) was injected via the tail vein. EPR imaging: All EPR images were acquired using an in-house built 750 MHz CW-EPR imager. Using the rapid magnetic field scan system, the fastest data acquisition time for 3D-EPR images is about 9 s for 50 ms field scanning (6 mT field scan) and 181 projections. MRI measurements: MRI of mouse heads was acquired using an MRmini scanner (MR Technology, Tsukuba, Japan) with a 0.5 T permanent magnet. Two-dimensional EPR slice images were co-registered to MRI anatomical images of examined mouse heads.RESULTS & DISCUSSION
The chemical structures of the MCP and AMCP imaging probes are shown in Fig. 1. After injecting mice with MCP and AMCP, the distribution of each probe in the mouse heads was visualized by EPR imaging in the sagittal and axial planes. These images were coregistered to pre-injection MR images of the same mice. The co-registered images shown in Fig. 2 confirm that both of the probes can pass through the BBB into the brain and that they have similar distributions within the brain. After penetration of the BBB, MCP retains its methyl ester bond without hydrolysis of the bond, and behaves as a hydrophobic nitroxide. In contrast, after crossing the BBB, AMCP is hydrolyzed in situ by esterase to become COP, a hydrophilic nitroxide (Fig. 1C). Serial EPR imaging of the mouse heads was obtained every 9 s after infusion of the probes. Pixel-based rate constants based on the pharmacokinetics of the reduction reactions of MCP and AMCP were calculated and mapped. These redox maps were then co-registered to the MR images (Fig. 3). The average rate constants of MCP and AMCP were calculated for selected regions of interest in the mouse heads. Outside the brain, the reduction rate constant for MCP (0.11 ± 0.02 min-1, n=3) was similar to that for AMCP (0.10 ± 0.03 min-1, n=3), which is the same as the reduction reactions of these nitroxides with ascorbic acid in an in vitro system. Within the brain, however, the reduction rate constant for AMCP (0.07 ± 0.02 min-1, n=3) was 7 times lower than that for MCP (0.52 ± 0.08 min-1, n=3), which suggests the ability of these two nitroxides to assess brain regions of different redox status. The reduction rates of nitroxides in vivo depend on levels of the endogenous antioxidants ascorbic acid and glutathione2. Therefore, the sites of MCP and AMCP visualization are related to antioxidant levels at those locations, according to redox status.CONCLUSION
Brain redox imaging using BBB-permeable MCP and AMCP clearly showed the association between each nitroxide probe and regions of particular redox status within the brain. This method could be of use for evaluating redox status of the brain in oxidative brain disease by EPR imaging and MRI.Acknowledgements
This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI grant Number 19K07920.References
1. Sano H, et al. A new nitroxyl-probe with high retention in the brain and its application for brain imaging. Free Rad Biol Med. 2000; 28(6): 959-969. 2. Emoto MC, et al. Non-invasive mapping of glutathione levels in mouse brains by in vivo electron paramagnetic resonance (EPR) imaging: Applied to a kindling mouse model. Neurosci Lett. 2019; 690: 6-10.Figures
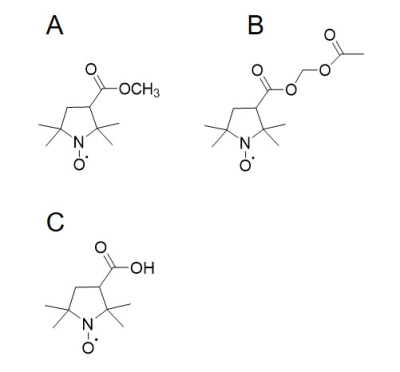
Chemical structure of the nitroxides used in this study. A; MCP, B; AMCP, C: COP.
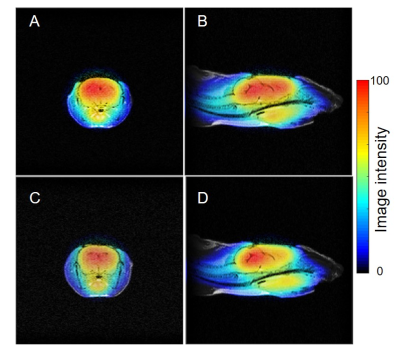
Co-registered images of mouse heads showed the distribution of MCP and AMCP. Axial direction; MCP:A, AMCP: C. Sagittal direction;MCP: B, AMCP: D.
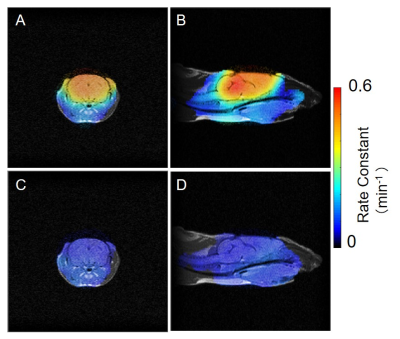
Co-registered images of redox maps by EPR and anatomical image by MRI for mouse heads. Axial direction; MCP:A, AMCP: C. Sagittaldirection; MCP: B, AMCP: D.
DOI: https://doi.org/10.58530/2022/4644