4643
High-resolution multimodal imaging of the embryonic and neonatal mouse1Human Informatics and Interaction Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan, 2Faculty of Engineering, University of Tsukuba, Tsukuba, Japan, 3Center for Information and Neural Networks (CiNet), Osaka University and National Institute of Information and Communications Technology (NICT), Osaka, Japan, 4Developmental Neuroscience Project, Department of Brain & Neurosciences, Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan, 5Division of Regenerative Medicine, Jikei University school of Medicine, Tokyo, Japan, 6Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
Synopsis
The potential of magnetic resonance microscopy is to image the multimodal function of the brain in small brain, such as embryo. In this study, we developed high resolution imaging of T1 image, T2 mapping, mean diffusivity, and neurite orientation dispersion by diffusion MRI in embryo and early postnatal (P07) in mouse. These values were different between embryo and early postnatal. Immunohistochemistry revealed distinct proliferation and maturation of neurons and astrocytes in these stages. These results indicate the possibility of high-resolution MR microscopy to detect the cellular maturation in development.
Purpose
High resolution magnetic resonance microscopy (MRM) with high field MRI is required to investigate the development of the brain structure and function. This study aims to develop high-resolution T2 and diffusion-weighted imaging, including apparent diffusion coefficients and neurite orientation dispersion and density imaging (NODDI), of mouse brain at embryonic and neonatal stages.Methods
AnimalsEmbryo (n=2) and postnatal (n=1) C57BL/6J mice were sacrificed at embryo day 16 (E16), embryo day 18 (E18) and postnatal day 7 (P07). A whole body of E16 was fixed by 4% paraformaldehyde (PFA) and immersed in Gd solution (Gadodiamide Hydrate, 5mM) after fixation. A whole body of E18 was fixed by PFA and immersed in PBS after fixation. Mouse at P7 was fixed through cardiac perfusion of PBS followed by PFA.
MRI experiment
MRI acquisition was conducted at Bruker 11.7 T vertical magnet with a volume coil. Multi-b value diffusion MRI images were acquired using a diffusion weighted spin-echo EPI sequence, TR/TE = 12500 / 24.6 ms, spatial resolution = 100 x 100 x 100 µm3, 50 slices, b-values = 0, 1000, 2000, 4000 s/mm2, and 22 averages. Multi-shot T2 images were acquired using multi-slice multi-echo with following parameters, resolution = 59 x 59 x 200 µm3/voxel, 38 slices, 16 effective TEs. T1 weighted images of E16 were acquired using 3D fast low angle shot (FLASH) such as TR/TE = 40 / 10 ms, FA = 90 degree, spatial resolution = 29 x 29 x 50 µm3, 10 averages, and reconstructed resolution = 15 x 15 x 50 µm3.
Immunostaining
Embryo and postnatal mice fixed for 1 hr in 4% PFA were equilibrated in 30% sucrose and embedded. Then, 20 µm slices cut in a cryostat. The following primary antibodies were used: rabbit anti-Brn2, rabbit anti-Sox9, mouse anti-NeuN. Images including DAPI staining were acquired using Stellaris confocal laser microscope.
Image processing
Orientation dispersion index (ODI) was calculated using NODDI toolbox1. Mean diffusivity (MD) was calculated using DSIstudio2. T2 relaxation time was calculated with following equation; S = A*exp(-TE/T2) + B, where A is a proton density (PD).
Results and Discussion
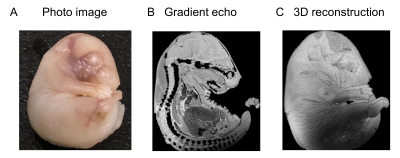
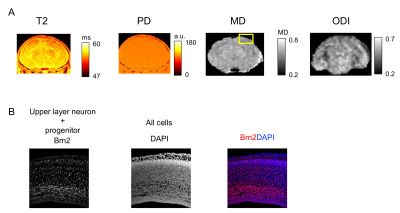
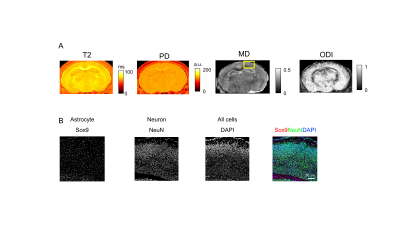
We established high resolution MR microscopy of embryo and early postnatal brain. T1-weighted image of the brain at E16 with Gd shows the fine contrast at high-resolution (15 x 15 x 50 µm3/voxel) (Fig. 1). We also investigated T2, proton density (PD), mean diffusivity (MD) of water and oriented dispersion index (ODI) (Fig. 2 and 3). T2 value at P07 was higher than that at E18 and MD is negatively proportional to T2 (Fig. 2 and 3). MD at P07 was lower than that at E18, while ODI at P07 was higher than E18. We also compared the distribution of neurons and astrocytes in the cortex (rectangle in the MD image in Fig 2 and Fig 3) using immunohistochemistry. The neurons and astrocytes were increased at P07. Astrocyte was not observed, and neurons were immature (Brn2 is a neuronal marker) at E18. Additionally, DAPI staining showed the dense immature cells, which did not express the neuronal/astrocyte markers, at E18. So, reduction of T2 and less restriction of water diffusion (MD) at E18 compared with P07 could be due to the dense immature cells. Additionally, lower ODI at E18 than that of P07 indicates the simpler distribution of neurites at E18. These results indicate that MR microscopy is useful to detect the spatial distribution of the cells during development of the brain.Acknowledgements
No acknowledgement found.References
1. Zhang et al, NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain, NeuroImage, 2012, Jul 16;61(4):1000-16. 2.
2. Fang-cheng Yeh, DSI Studio (Version 2021 May). (2021, May 15), Zenodo, http://doi.org/10.5281/zenodo.4764264.
Figures