4640
T2/T1-weighted MRI revealed the therapeutic role of microglial SMAD7 in a traumatic brain injury (TBI) mouse model
1Wuhan United Imaging Life Science Instrument Co., Ltd., China, Wuhan, China, 2Department of Neurosurgery, Tongji Hospital, Tongji Medical College,, Huazhong University of Science and Technology, Wuhan, China
Synopsis
We applied T2/T1-weighted MRI together with behavior, Hematoxylin&Eosin(HE)and immunohistochemistry fluorescence staining (IHC) to evaluate the role of microglial SMAD7, a novel therapeutic approach, in a TBI mouse model. The consistency between MRI results and behavior and myelin and neurnal marker outcomes suggest that the protective role of over-expression of microglial SMAD7 in TBI.
INTRODUCTION
Traumatic brain injury (TBI) is the most detrimental cause of death and disability among diseases of central nerve system. In addition, the secondary injury of TBI, including neuroinflammation, edema and demyelination etc., worse clinical outcomes. The pro-inflammatory characteristic of microglia during TBI may lead to the secondary injury of TBI. Therefore, targeting the regulating mechanisms of microglia polarization post TBI is crucial for new therapeutic strategies. In particular, transcription factor SMAD7 negatively regulates NF-kb signaling, a classic inflammation related signaling, that participates in microglial induced neuroinflammation in the process of TBI [1]. Hence, we reckon that overexpression of SMAD7 may affect its pro-inflammatory properties of microglial and thus alleviate the neuroinflammation which result in better neuronal-vascular unit recovery.MRI has been proven to be a powerful diagnostic and prognostic tool in numerous diseases, including TBI, and can play an important role in longitudinal studies that are needed to understand the dynamic nature of brain injury in TBI [2,3]. Amongst, T2-weighted images can identify hyper-intensive abnormalities, i.e. the TBI-induced edema while T1-weighted images show structure damage details by TBI. Thus, we aimed to apply T2/T1-weighted images incorporation with behavior and gold standard neuronal structures to evaluate the therapeutic role of microglial SMAD7 in a TBI mouse model.
METHODS
MR Instruments: All MR experiments were performed in a horizontal 15cm-inner-diameter 9.4T magnet (UIH, P.R.C.), with a maximum gradient strength of 800mT/m and a slew rate up to 2000T/m/s 7cm-inner-diameter gradient insert (UIH, P.R.C.). A single-loop coil with a 15mm-inner-diameter was used as transmitter and receiver.Animals studies: The study was approved by the local Committee for the Care of Animals. In total, 40 male C57BL/10ScNJ mice (8-10 weeks old; 24.2±1.7 g) were used. A typical experimental layout was followed as in Figure 1. On day 0, Adeno-associated virus (AAV-vector) with/without specifically over-express microglial SMAD7 (AAV-smad7 OE) were injected for the evaluation of the impact of microglial SMAD7 over-expression on post-TBI recovery.
Controlled cortical impact (CCI) model of TBI in mice: On day 30, TBI model was performed according to a well-established method [4,5]. In brief under intraperitoneally chloral hydrate (400 mg/kg body weight) administration, all mice were subjected to a unilateral, moderately controlled cortical impact (CCI) of 2.0mm depth at 3.5m/s and 500ms dwell time using the TBI 0310 with a hard stop Bimba cylinder.
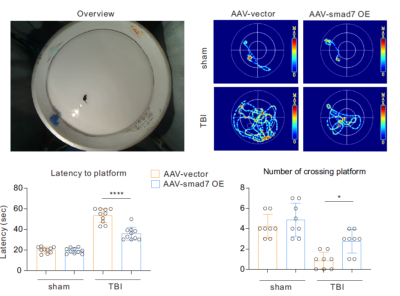
Behavior test: After day 50 and a couple of days before the scheduled MRI scans, mice destined to be scanned together with others underwent the Morris water maze (MWM) training for 5 days and for examining latency to platform and number of platform crossing, as previously described [6]. All mice were monitored by a video tracking system directly above the water tank as data are measured using Ethovision software in a computer.
MR study: Three mice from each group, namely sham, TBI with AAV-vector only (AAV-vector) and TBI with AAV-smad7 OE (AAV-smad7 OE), were anaesthetized with 0.1mL 10% chloral hydrate was intraperitoneally injected. Once animal heads were secured with two ear pieces and one ear bar. Once the coil was placed on top of the head and well-secured, the whole setting was centered to the MRI scanner. T1-weighed images were acquired for detailed studctures (TE/TR=7.5/1025ms, field of view (FOV): 9×14mm2, slices: 25×0.6mm, matrix size: 169×264, ETL: 3, bandwidth=200Hz/pixel, NEX=30). T2-weighed images were performed to investigate the edema area, parameters were TE/TR:51.12/4000ms, FOV:9×14mm2, slices:25×0.6mm, matrix size: 113×176, ETL:11, bandwidth=200Hz/pixel, NEX=30.
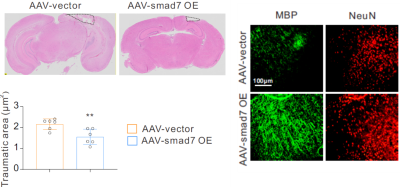
Immunohistology: At the end of experiments, all mice were sacrificed and prepared accordingly [7] and their neuronal structures were assessed by Hematoxylin&Eosin(HE)and immunohistochemistry fluorescence staining (IHC).
Analysis and Statistics: Images were analyzed using U_VIEWER (UIH, P.R.C.) and ImageJ.The infarct volume was calculated by summing the infarct area per slice by multiplying the slice thickness. The statistics results were performed using Graphpad Prism 9.1.0 and considered to be significant when p<0.05.
RESULTS AND DISCUSSION
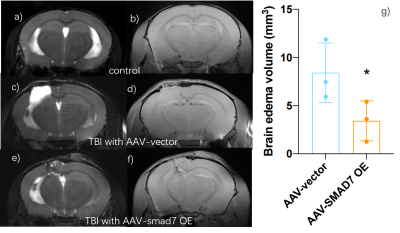
60 days after AAV-vector and 30 days after TBI, sham-operated AAV-vector mice did not show any brain abnormalites in T2-weighted and T1-weighted images (Figure 2a and 2b). However, T2-weighted images of those TBI mice with AAV vector have edema with 8.05 ± 3.52 mm3 when comparing to brain edema volume of those AAV-SMAD7 OE mice was 3.42 ± 2.08mm3 (p < 0.05; n = 3 per group). Overall, MRI images showed that microglial SMAD7 overexpression significantly reduced traumatic injury volume as well as edema volume post TBI (Figure 2). Rotarod and Morris water maze results were performed and we found significant improvement of cognitive behavior in SMAD7 over-expression TBI mice (Figure 3). In addition, we evaluated the re-myelin level as well as the neuronal cell density after TBI in selected mice from these MRI-measured mice and other non-MRI-measured ones, higher density of myelin and neuronal cells were also observed in SMAD7 over-expression mice, as shown in Figure 4 and 5. Overall, the protective role of SMAD7 after TBI was noticeable in MRI results (Figure 2), consistent with the behavior and gold standard immuno-staining results. Taken together, T2/T1-weighted MRI results are in line with the corresponding behavior and histopathology determinations that SMAD7 overexpression in microglia induced protective role post TBI, which may provide potential therapeutic target for TBIAcknowledgements
The present study was supported by the Natural Science Foundation of Hubei province (grant no. WJ2019Z008), the Natural Science Foundation of Tongji Hospital (grant no. 2020JZKT651), the 3551 Guanggu Talent Program of Hubei province and Wuhan United-Imaging Life Science Instrument Co. Ltd.References
1. Patel RK, Prasad N, Kuwar R, Haldar D, Abdul-Muneer PM “Transforming growth factor-beta 1 signaling regulates neuroinflammation and apoptosis in mild traumatic brain injury” Brain Behav Immun 2017 Aug;64:244-258 DOI: 10.1016/j.bbi.2017.04.012
2. Hetherington H, Bandak A, Ling G, Bandak FA. “Advances in imaging explosive blast mild traumatic brain injury.” Handb Clin Neurol. 2015;127:309-18. doi: 10.1016/B978-0-444-52892-6.00020-9.
3. Lodygensky GA, Inder TE, Neil JJ “Application of magnetic resonance imaging in animal models of perinatal hypoxic-ischemic cerebral injury” Int J Dev Neurosci. 2008 Feb;26(1):13-25. doi: 10.1016/j.ijdevneu.2007.08.018.
4. Yao X, Liu S, Ding W, Yue P, Jiang Q, Zhao M, Hu F, Zhang H “TLR4 signal ablation attenuated neurological deficits by regulating microglial M1/M2 phenotype after traumatic brain injury in mice.” J Neuroimmunol. 2017 Sep 15; 310:38-45.
5. Menichetti A, Bartsoen L, Depreitere B, Vander Sloten J, Famaey N. “A Machine Learning Approach to Investigate the Uncertainty of Tissue-Level Injury Metrics for Cerebral Contusion.” Front Bioeng Biotechnol. 2021;9:714128
6. Barnhart CD, Yang DR, Lein PJ “Using the Morris Water Maze to Assess Spatial Learning and Memory in Weanling Mice” PlosOne 2015. DOI:10.1371/journal.pone.0124521
7. Ibrahim Fathi, Takehiro Imura, Akiko Inagaki , Yasuhiro Nakamura , Ayman Nabawi , Masafumi Goto. “Decellularized Whole-Organ Prevascularization: A Novel Approach for Organogenesis.” Front Bioeng Biotechnol. 2021,9:756755
Figures