4639
Correlating Raised Intracranial Pressure with Increased Brain Motion in a Ovine Model1Department of Anatomy and Medical Imaging, Faculty of Medical and Health Sciences & Centre for Brain Research, University of Auckland, Auckland, New Zealand, 2Auckland Bioengineering Institute, Auckland, New Zealand, 3Mātai Medical Research Institute, Gisborne, New Zealand, 4Department of Physiology, Faculty of Medical and Health Sciences, University of Auckland, Auckland, New Zealand
Synopsis
Heartbeat-driven brain motion has historically remained limited to the domain of research. Such motion may provide a window into conditions that alter the brain pressure where direct, invasive measurements are difficult to justify. Amplified MRI (aMRI) is a recently developed method which amplifies subtle brain motion, providing high temporal resolution and high contrast ‘videos’ of brain motion patterns. In an ovine model of elevated intracranial pressure we show preliminary data that aMRI can be used to detect changes in brain motion associated with pressure, indicating potential to provide non-invasive insight into the mechanical changes in patients with altered intracranial pressure.
Introduction
Heartbeat-driven motion in brain tissue is a well established phenomenon1,2, but its observation has historically remained limited to the domain of research, largely due to difficulty in adapting standard motion-sensitive MRI sequences for the exceptionally small tissue displacements of interest2,3.However, recent advances in MRI techniques have resulted in improvements in our ability to rapidly observe and inspect these motions4-7. These patterns of motion could provide a non-invasive window into the mechanical conditions inside a patient’s brain, guiding clinical diagnosis and intervention, especially in patients with chronic conditions where repeated, direct, invasive measurements are difficult to justify.
3D amplified MRI (or aMRI) is a method which amplifies subtle brain motion4-7, providing high temporal resolution and high contrast ‘videos’ of brain motion. These visualizations have shown promise in detecting subtle brain motion differences in CSF disorders such as Chiari Malformation6.
Methods
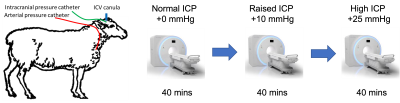
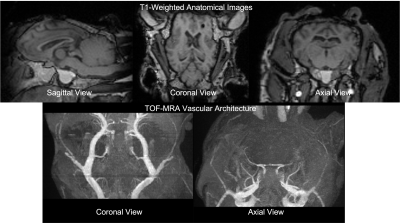
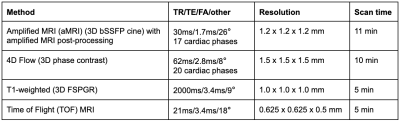
Animal model: All animal experiments were approved by the University of Auckland’s Animal Ethics Committee. All experiments were performed in accordance with the guidelines and regulations of the Ethics committee and these comply with the ARRIVE guidelines. Using previously developed procedures8,9, intracerebroventricular and subdural catheters were surgically placed in anesthetised sheep for the infusion of fluid to increase ICP and for the measurement of ICP respectively. The anesthetised and ventilated sheep were transferred to the MRI scanner and positioned on their right side with the head stabilised within the head coil. During elevated pressure periods, saline was continuously infused through the ventricular catheter into the ventricles to maintain a steady ICP offset. Motion sensitive MRI images were acquired at three distinct ICP levels - unmodified baseline (+0 mmHg), moderate elevation (+10 mmHg) and high elevation (+25 mmHg) (Figure 1).Image Acquisition: All scans were acquired on a 3T MAGNETOM Skyra system (Siemens Healthcare, Erlangen, Germany) using a 32-channel head coil. T1-weighted MRI, cine 3D bSSFP (the base acquisition for aMRI), Time-of-Flight (TOF) MRI, and 4D flow MRI (Table 1) along with other conventional sequences (combined scan time of approximately 40 min) were acquired at each ICP level.
Data Visualisation and Analysis:
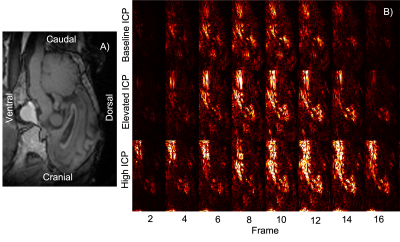
The cine bSSFP data was amplified with a 25x global amplification factor, and difference maps were calculated across the cardiac frames. The difference maps were generated by subtracting each cardiac frame from the first frame, providing a visualization of the regions where motion had resulted in significant changes in pixel intensity.
Results
Elevated ICP was found to alter patterns of brain tissue motion (Figure 3). In our ovine model, elevated ICP was positively correlated with increased brain tissue displacement during the cardiac cycle. This pattern of increased motion was consistent across most of the brain, particularly in the lower regions of the brain and in the brainstem where cardiac-driven motion is most easily observed.Discussion
Cardiac-linked brain motion has the potential to allow for non-invasive measurement of ICP which would be revolutionary for the clinical management of patients with intracranial hypertension. Previous studies have examined the link between elevated ICP and brain tissue motion. Saindaine et al3 used DENSE MRI to compare the brain tissue motion in patients with chronically elevated ICP before and after a lumbar puncture (LP) procedure. The study found a link between elevated ICP and increased pontine displacement, but they were unable to accurately predict ICP from this metric. These results are consistent with the results presented here, providing a proof of concept for aMRI as an alternate, non-phase contrast method of detecting and quantifying this behaviour.In Alperin’s paper on ‘ICP measurement’10, they cast the net further to use their model to differentiate between high and low ICP in patients with pseudotumour cerebri (Idiopathic Intracranial Hypertension - IIH) using their MRI measures of volume (derived from blood and CSF flow) and pressure changes (derived from CSF velocities) to estimate ICP. This is based on a single MRI-derived elastance coefficient – which they assume is constant.
While providing an important basis for estimating ICP, the current models proposed by Alperin treat the entire brain as a homogeneous unit and are not able to account for regional or temporal variations in actual tissue compliance. Our own preliminary data from aMRI indicate regional variations in tissue displacement (and hence in regional elastance). Using aMRI, our future studies will move beyond a lumped model of cerebral elastance and incorporate metrics of tissue displacement into the model to account for regional effects which we hypothesise are important for a more accurate ICP measurements.
Conclusion
aMRI is able to detect changes in cardiac-driven brain tissue pulsations that arise due to changes in ICP. This indicates a potential for aMRI techniques to provide non-invasive insight into the mechanical conditions in a patient’s brain.Further work will involve drawing insight into the mechanisms underlying this behaviour through the analysis of 4D flow images and, in the long term, the development of a mechanical model of the brain to allow brain motion to be used as an index for patient ICP.
Acknowledgements
This work was supported by the Royal Society of New Zealand Marsden Fund. We are grateful to Anna-Maria Lydon at Centre for Advanced MRI (CAMRI), at the University of Auckland for support with the MRI protocol; and to the VJU at the University of Auckland for support with the sheep study.References
Soellinger, M., Rutz, A. K., Kozerke, S., & Boesiger, P. (2009). 3D cine displacement-encoded MRI of pulsatile brain motion. Magnetic Resonance in Medicine, 61(1), 153–162. https://doi.org/10.1002/mrm.21802
Poncelet, B. P., Wedeen, V. J., Weisskoff, R. M., & Cohen, M. S. (1992). Brain parenchyma motion: Measurement with cine echo-planar MR imaging. Radiology, 185(3), 645–651. https://doi.org/10.1148/radiology.185.3.1438740
Saindane, A. M., Qiu, D., Oshinski, J. N., Newman, N. J., Biousse, V., Bruce, B. B., Holbrook, J. F., Dale, B. M., & Zhong, X. (2018). Noninvasive Assessment of Intracranial Pressure Status in Idiopathic Intracranial Hypertension Using Displacement Encoding with Stimulated Echoes (DENSE) MRI: A Prospective Patient Study with Contemporaneous CSF Pressure Correlation. American Journal of Neuroradiology, 39(2), 311–316. https://doi.org/10.3174/ajnr.A5486
Holdsworth, S. J., Rahimi, M. S., Ni, W. W., Zaharchuk, G., & Moseley, M. E. (2016). Amplified magnetic resonance imaging (aMRI): Amplified MRI (aMRI). Magnetic Resonance in Medicine, 75(6), 2245–2254. https://doi.org/10.1002/mrm.26142
Terem, I., Ni, W. W., Goubran, M., Rahimi, M. S., Zaharchuk, G., Yeom, K. W., Moseley, M. E., Kurt, M., & Holdsworth, S. J. (2018). Revealing sub-voxel motions of brain tissue using phase-based amplified MRI (aMRI): Terem et al. Magnetic Resonance in Medicine, 80(6), 2549–2559. https://doi.org/10.1002/mrm.27236
Terem, I., Dang, L., Champagne, A., Abderezaei, J., Pionteck, A., Almadan, Z., Lydon, A.-M., Kurt, M., Scadeng, M., & Holdsworth, S. J. (2021). 3D amplified MRI (aMRI). Magnetic Resonance in Medicine, 86(3), 1674–1686. https://doi.org/10.1002/mrm.28797
Abderezaei, J., Pionteck, A., Terem, I., Dang, L., Scadeng, M., Morgenstern, P., Shrivastava, R., Holdsworth, S. J., Yang, Y., & Kurt, M. (2021). Development, calibration, and testing of 3D amplified MRI (aMRI) for the quantification of intrinsic brain motion. Brain Multiphysics, 100022. https://doi.org/10.1016/j.brain.2021.100022
Vari, S., Guild, S.-J., George, B., & Ramchandra, R. (2021). Intracranial baroreflex is attenuated in an ovine model of renovascular hypertension. Scientific Reports, 11(1), 5816. https://doi.org/10.1038/s41598-021-85278-3
Guild, S.-J., Saxena, U. A., McBryde, F. D., Malpas, S. C., & Ramchandra, R. (2018). Intracranial pressure influences the level of sympathetic tone. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 315(5), R1049–R1053. https://doi.org/10.1152/ajpregu.00183.2018
Alperin, N. J., Lee, S. H., Loth, F., Raksin, P. B., & Lichtor, T. (2000). MR-Intracranial Pressure (ICP): A Method to Measure Intracranial Elastance and Pressure Noninvasively by Means of MR Imaging: Baboon and Human Study. Radiology, 217(3), 877–885. https://doi.org/10.1148/radiology.217.3.r00dc42877
Figures