4629
A Systematic Cartilage Surface Segmentation Method for Cartilage Thickness Mapping
Yongcheng Yao1, Dόnal G. Cahill1, James F. Griffith1, and Weitian Chen1
1The Chinese University of Hong Kong, New Territories, Hong Kong
1The Chinese University of Hong Kong, New Territories, Hong Kong
Synopsis
Thickness mapping techniques are important in the management of osteoarthritis. Since most thickness mapping approaches require two surfaces as inputs, surface segmentation is important for articular cartilage thickness mapping. In this work, a systematic approach is proposed for automatic articular cartilage surface segmentation from a volume segmentation mask, which includes cartilage surface reconstruction, body surface construction, surface normal estimation, directional voxels searching, restricted surface dilation, and surface close operation. The proposed method can be used for reliable cartilage thickness mapping.
Introduction
Osteoarthritis (OA) is one of the main causes of joint disability1. Measurements of progressive articular cartilage loss at different clinical stages of OA is important in the management of OA2–10. Thickness mapping techniques3,8,9 have been developed to investigate the pattern of articular cartilage loss. Since the mainstream thickness mapping methods9–11require the inner and outer surface as inputs, surface segmentation is a vital step for most cartilage thickness mapping processes. In this work, we propose a systematic approach for automatic articular cartilage surface segmentation from a single volume segmentation mask. Together with existing three-dimensional volume segmentation techniques12, the proposed method paves the way for accurate and robust articular cartilage thickness mapping.Methods
Prior to applying the surface segmentation, surface reconstruction is applied to the volume segmentation masks to represent the surface as a triangular mesh which consists of vertices and faces. Then, the mesh is segmented into inner (i.e., bone-cartilage interface) and outer surfaces via the proposed coarse-to-fine surface segmentation pipeline (Figure 1).At the coarse segmentation stage, two primary set of vertices on the inner and outer surfaces are formed. First, a body surface of the cartilage volume is constructed (Figure 1a). Second, for each vertices on the body surface, surface normal estimation (Figure 1b) and orientation correction (Figure 1c) are carried out. More specifically, normal vectors are estimated by Singular Value Decomposition, and an efficient algorithm (Figure 1d) is developed for orientation correction. The outputs of this step are normal vectors on the body surface pointing to the inner surface. Finally, with a proposed directional searching algorithm and the orientation information from surface normals, vertices on the inner surface can be identified. Similarly, the inverted surface normals can locate vertices on the outer surface.
At the fine-grain segmentation stage, restricted surface dilation and surface close are proposed to fine-tune the meshes of inner and outer surfaces. First, a primary mesh consisting of vertices from the coarse segmentation step is formed. Sequentially, the meshes of the outer and inner surfaces are expanded via restricted surface dilation in order. Then, the surface close operation (Figure 1e) can further adjust the mesh of inner surface (Figure 1f). Analogous to the morphological close operation, the proposed surface close is a surface dilation followed by surface erosion. The surface dilation extends the surface by one layer of faces along the edge. The surface erosion, on the other hand, trims the surface by one layer of faces on the edge region. Considering the scenario where erroneous holes exist in the segmented surface, the surface close operation can add missing faces back while retaining the surface edge. Finally, the outer surface is fine-tuned through restricted surface dilation (Figure 1g).
Data sets used in this study were from the publically available Osteoarthritis Initiative (OAI) project. The data sets were acquired using a 3D DESS pulse sequence with resolution 0.7*0.3646*0.3646 millimetre at 3.0T.
Results
Figure 2 shows typical results of articular cartilage surface segmentation using the proposed method. Note our method can detect inner and outer surfaces effectively, which is essential for accurate articular cartilage thickness measurement. In Figure 3, the surface segmentation outputs are fed into an in-house thickness mapping algorithm to generate thickness maps. Note the spatial variation of cartilage thickness across the bone-cartilage interface is clearly visualized in the thickness map.Discussion
The major novelty of the proposed method is an automatic and accurate surface segmentation from an articular cartilage mask. In humans, with prior knowledge of the shape and orientation of a specific cartilage type, it is relatively easy to differentiate the bone-cartilage interface from the outer surface. However, from a machine point of view, the information encoded in a three dimensional volume mask is not enough for surface segmentation. In this work, we encode prior knowledge in a reference vector pointing from the outer surface to the inner surface. A seed region is first defined as the initial area of the orientation correction process. Starting from a vertex in the seed region which has surface normal parallel to the reference vector, the surface normals within the adjacent area of seed vertex are rectified. Then vertices on the peripheral circle become new seed vertices, and the next round of rectification begins. As demonstrated (Figure 1d), the proposed algorithm can flip all normal vectors to the bone-cartilage interface.The combination of restricted surface dilation and surface close is tailor-made for articular cartilage thickness mapping. In regions with severe cartilage loss, there may be only one or no layer of voxels in the volume mask. In such a case, the surface segmentation becomes challenging. The proposed restricted surface dilation and surface close ensure the robustness and accuracy of our method. When extensive full-thickness cartilage loss is present, the algorithm can identify more than 99% area of the inner surface (Figure 2b).
Conclusion
We proposed a pipeline for automatic articular cartilage surface segmentation from a single volume segmentation mask, which includes cartilage surface reconstruction, body surface construction, surface normal estimation, directional voxels searching, restricted surface dilation, and surface close operation. Results show that the proposed method is robust against cartilage shape variation and cartilage loss.Acknowledgements
This study was supported by a grant from the Innovation and Technology Commission of the Hong Kong SAR (Project MRP/001/18X).References
1. Hunter, D. J., March, L. & Chew, M. Osteoarthritis in 2020 and beyond: a Lancet Commission. The Lancet 396, 1711–1712 (2020).2. Wirth, W. et al. Spatial patterns of cartilage loss in the medial femoral condyle in osteoarthritic knees: Data from the osteoarthritis initiative. Magn. Reson. Med. 63, 574–581 (2010).
3. Williams, T. G. et al. Measurement and visualisation of focal cartilage thickness change by MRI in a study of knee osteoarthritis using a novel image analysis tool. Br. J. Radiol. 83, 940–948 (2010).
4. Buck, R. J., Wirth, W., Dreher, D., Nevitt, M. & Eckstein, F. Frequency and spatial distribution of cartilage thickness change in knee osteoarthritis and its relation to clinical and radiographic covariates – data from the osteoarthritis initiative. Osteoarthritis Cartilage 21, 102–109 (2013).
5. Eckstein, F. et al. Brief Report: Cartilage Thickness Change as an Imaging Biomarker of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 67, 3184–3189 (2015).
6. Erhart-Hledik, J. C., Favre, J. & Andriacchi, T. P. New insight in the relationship between regional patterns of knee cartilage thickness, osteoarthritis disease severity, and gait mechanics. J. Biomech. 48, 3868–3875 (2015).
7. Favre, J., Scanlan, S. F., Erhart-Hledik, J. C., Blazek, K. & Andriacchi, T. P. Patterns of Femoral Cartilage Thickness are Different in Asymptomatic and Osteoarthritic Knees and Can be Used to Detect Disease-Related Differences Between Samples. J. Biomech. Eng. 135, 101002 (2013).
8. Favre, J. et al. Anatomically Standardized Maps Reveal Distinct Patterns of Cartilage Thickness With Increasing Severity of Medial Compartment Knee Osteoarthritis. J. Orthop. Res. 35, 2442–2451 (2017).
9. MacKay, J. W. et al. Three‐Dimensional Surface‐Based Analysis of Cartilage MRI Data in Knee Osteoarthritis: Validation and Initial Clinical Application. J. Magn. Reson. Imaging 52, 1139–1151 (2020).
10. Favre, J. et al. Analyzing Femorotibial Cartilage Thickness Using Anatomically Standardized Maps: Reproducibility and Reference Data. J. Clin. Med. 10, 461 (2021).
11. Carballido-Gamio, J. & Majumdar, S. Atlas-based knee cartilage assessment: Atlas-Based Knee Cartilage Assessment. Magn. Reson. Med. 66, 575–581 (2011).
12. Gan, H.-S., Ramlee, M. H., Wahab, A. A., Lee, Y.-S. & Shimizu, A. From classical to deep learning: review on cartilage and bone segmentation techniques in knee osteoarthritis research. Artif. Intell. Rev. 54, 2445–2494 (2021).
Figures
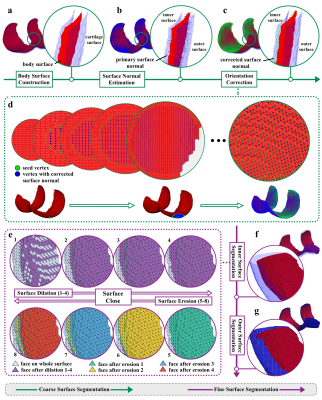
Figure 1. Coarse-to-fine Surface Segmentation. (a) Body surface construction. (b) Surface normals estimation on the body surface. (c) Orientation-corrected surface normals on the body surface. (d) Surface normals orientation correction. (e) Surface close operation. (f) Segmented inner surface. (g) Segmented outer surface.
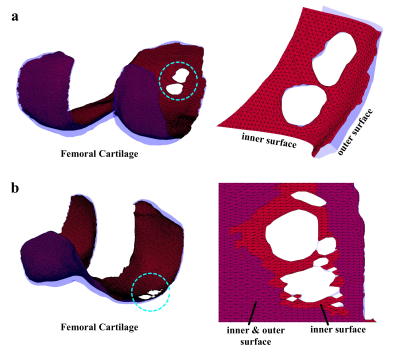
Figure 2. Examples of Cartilage Surface Segmentation. (a) A Femoral cartilage with localized full-thickness cartilage loss. (b) A Femoral cartilage with more extensive full-thickness cartilage loss.
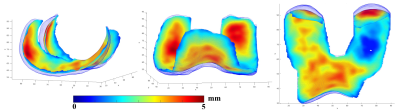
Figure 3. Examples of Cartilage Thickness Maps.
DOI: https://doi.org/10.58530/2022/4629