4610
Altered dynamic cerebral perfusion during recovery in right hemisphere subcortical stroke1The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China, 2GE Healthcare MR Research, Beijing, China, Beijing, China
Synopsis
To quantify cerebral blood flow (CBF) dynamic evolution and the relationship with clinical outcomes during recovery in right subcortical stroke patients,ASL and neurological examinations were performed repeatedly in 25 patients at 4 time points and 26 healthy controls (HCs) once.One-way Within-Subjects ANOVA and two sample test,pearson correlation analyses were performed.Compared with HCs, patients displayed continuous crossed cerebellar diaschisis.Increased CBF of contralesional sensorimotor cortex in the subacute and chronic are vital for recovery.Patients showed dynamic changes of CBF in contralesional cerebellum superior and occipital lobe,which were negatively correlated with memory.Findings provide a theoretical basis for functional impairment and recovery after stroke.
Purpose
Nerve injury and repair coexist during the first few months after stroke onset in patients, which are closely related to blood perfusion (Sandvig I et al., 2018). Restoring blood circulation (Koh et al., 2017) and coherent, ordered neural activity (Siegel et al., 2016) are important factors in stroke recovery. Dynamic changes in cerebral perfusion levels during recovery and the relationships with clinical outcomes are still unclear. We aimed to use 3D-pcASL to investigate cerebral blood flow (CBF) dynamic evolution and the relationship between abnormal CBF values and clinical outcomes during recovery in right hemisphere subcortical stroke patients.Method
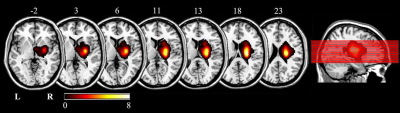
A total of 25 right-handed patients with right motor pathway subcortical stroke infarction and 26 right-handed HCs were enrolled. The first-onset stroke patients manifested motor or sensory perception deficits with a single lesion (Figure 1) involving basal ganglia and neighboring regions of right hemisphere enrolled within 7 days of stroke onset. A series of neurological examinations and MR scans were performed on stroke patients and HCs. Stroke patients were scanned and neurological assessed 4 times, i.e., 1 week, 1 month, 3 months and 6 months after stroke. HCs underwent MR scan and neurological examinations once to establish the average level of normal population. Neurological examinations included the National Institutes of Health Stroke Scale (NIHSS), motor function(the Simplified Fugl-Meyer Assessment Scale (FMA)), episodic memory (RAVLT) and digital working memory(n-back task). The imaging data were acquired using GE Discovery MR 750 3.0 Tesla MR scanner. The perfusion imaging was acquired using a 3D-pcASL sequence with background suppression using the following parameters: TR/TE = 5025/11.1ms; post label delay = 2025ms; FOV = 240 mm× 240 mm; spiral in readout of 8 arms with 512 sample points; FA = 111°; matrix = 128×128; slice thickness = 3.0 mm, no gap; 48 axial slices, number of excitations = 3, and 1.9 mm × 1.9 mm in-plane resolution.Statistical Analyses
The study performed cross-sectional analyses to investigate dynamic differences of CBF between HCs and stroke patients at different TPs after infarction. To further explore patients’ dynamic change pattern of CBF, one-way repeated measures analysis of variance (Within-Subjects ANOVA) was applied in longitudinal analysis. Regions with significantly abnormal CBF in the repeated measures were used for post hoc analyses in stroke patients and HCs. Pearson correlation analyses were performed to explore relationships between abnormal CBF values with neurological examinations over time in patients.
Results
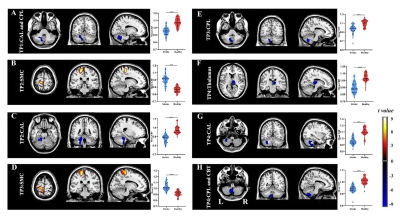
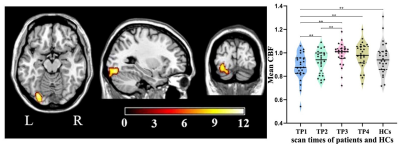
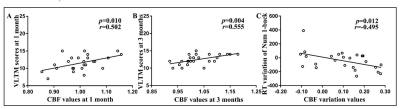
Compared with HCs, stroke patients displayed crossed cerebellar diaschisis (CCD) in the acute period and persisted to chronic phase (Figure 2ACEGH). Significant hypoperfusion in ipsilesional thalamus was only observed in the chronic period (Figure 2F). Of note, the significantly increased CBF in the subacute and chronic period were located in contralesional sensorimotor cortex (SMC) (Figure 2BD). There were significantly positive correlations between the increased CBF values of contralesional SMC at 1 month and VLTM scores at 1 month, as well as at 3 months(Figure 4AB). Further investigations showed the dynamic change pattern of CBF in stroke patients decreased at first, then increased over time, with peak at 3 months, followed by a slowly declining, which located in contralesional SCP and OCC (Figure 3) that associated with digital working memory(Figure 4C).Discussion
Stroke patients displayed crossed cerebellar diaschisis (CCD) in the acute period and persisted to chronic phase. Diaschisis is due to hemodynamic dysfunction, delayed neuronal death, inhibition of neural pathway in functionally connected areas which are remote from infarct lesion, as a result, leading to a wide-spread disconnection of brain networks. CBF in the contralesional sensorimotor cortex (SMC) appeared to increase at 1 month in stroke patients and the range of increased CBF at 3 months had become broader, which were significantly positive correlations with VLTM scores at 1 month, as well as at 3 months.Increased CBF of contralesional SMC in the subacute and chronic are vital components for motor function and episodic memory recovery. The study showed that patients' motor, episodic memory and digital working memory were impaired in the acute phase and significant improvement in subacute and chronic phase, which coexist the dynamic change pattern of CBF in contralesional SCP and OCC, suggesting ischemic injury and nerve repair can lead to functional impairment and reorganization in brain areas of distant lesion that were accompanied clinical symptoms.Conclusion
These findings might facilitate our understanding of the dynamic change pattern of CBF and its relationships with clinical outcomes in stoke patients, which provide a theoretical basis for the study of the mechanism of functional impairment and recovery after stroke.Acknowledgements
We are grateful to participants and their families for generously supporting the study. This study was supported by the National Natural Science Foundation of China (Nos. 81871327), The Young Talents Promotion Program of Henan Province (No. 2021HYTP012). General Program Cultivation Foundation of Zhengzhou University (No. JC21854044).References
1. Sandvig, I., Augestad, I. L., Håberg, A. K., and Sandvig, A. (2018). Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur J Neurosci. 47(12), 1414–1428. doi:10.1111/ejn.13959
2. Koh, S. H., and Park, H. H. (2017). Neurogenesis in Stroke Recovery. Translational stroke research. 8(1), 3–13. doi:10.1007/s12975-016-0460-z
3. Siegel, J. S., Ramsey, L. E., Snyder, A. Z., Metcalf, N. V., Chacko, R. V., Weinberger, K., et al. (2016). Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc Natl Acad Sci U S A. 113(30), E4367–E4376.doi:10.1073/ pnas. 1521083113
Figures