4604
Reduced Blood Brain Barrier Water Exchange and Cerebral Blood Flow in CARASIL Patients Revealed by Perfusion MRI: a pilot study1Institute of Biophysics, Chinese Academy of Sciences, Beijing, China, 2University of Chinese Academy of Sciences, Beijing, China, 3Department of Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 4Department of Neurology, Xuanwu Hospital of Capital Medical University, National Clinical Research Center for Geriatric Diseases, Beijing, China, 5Mark & Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States, 6The Innovation Center for Excellence in Brain Science and Intelligence Technology, Chinese Academy of Sciences, Beijing, China, 7Siemens Shenzhen Magnetic Resonance Ltd, Shenzhen, China, 8Beijing Institute for Brain Disorders, Beijing, China
Synopsis
We applied 3D diffusion-prepared pseudo-continuous arterial spin labeling (DP-pCASL) to evaluate water exchange rate (kw) of blood brain barrier (BBB) and its association with clinical characteristics of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) patients. The CARASIL group showed significantly decreased kw and cerebral blood flow (CBF) compared with healthy controls. The association between kw with age, CBF and white matter hyperintensity volumes (WMH) found in CARASIL patients suggest that kw could be a potential non-contrast imaging biomarker for BBB dysfunction in cerebral small vessel disease.
Introduction
Cerebral small vessel disease (cSVD) is one of the most important causes of stroke and dementia1. As a rare genetic form of cSVD, cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) mainly affects brain arterioles and capillaries leading to cerebral hypoperfusion, blood-brain barrier (BBB) damage and lacunar infarcts2. The accurate quantification of BBB function is important to CARASIL clinical assessment. Positron emission tomography (PET) and dynamic contrast-enhanced (DCE) MRI are most commonly used in previous studies3,4. However, they are not suitable for detecting subtle BBB dysfunction and require injection of radioactive tracers or contrast agents. Recently, 3D diffusion prepared pseudo-continuous arterial spin labeling (DP-pCASL) MRI5 has been used to evaluate BBB function by treating water as an endogenous “tracer” to noninvasively evaluate the water exchange rate (kw) across BBB. The purpose of our study is to evaluate kw using 3D DP-pCASL sequence and to explore its correlations with other clinical characteristics in CARASIL patients.Methods
Subjects: In this study, 10 CARASIL patients (40.7±14.6 years, 3 females; 7 of 10 are symptomatic, 47.1±11.8 years, 2 females) and 7 age-matched healthy controls (46.0±13.6, 2 females) were recruited. Both the patients and control group underwent MRI scanning on a 3T MRI system (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with the same imaging protocol.Imaging parameters: The imaging protocol included 3D DP-pCASL, multi-delay pCASL, T2-FLAIR and T1-MPRAGE sequences. For DP-pCASL sequence with background suppressed 3D gradient and spin echo (GRASE) readout5 : resolution=3.5×3.5×8 mm3,12 axial slices, 2 post-labeling delays (PLDs) = 900/1800 ms, b= 0/14 s/mm2 for PLD 900ms and 0/50 s/mm2 for PLD 1800ms, scan time=10min. For multi-delay pCASL scan using 5-delay PLDs with background suppressed 3D GRASE readout7: resolution = 2.5×2.5×3mm3, PLDs= 0.5/1/1.5/2/2.5 s, labeling pulse duration= 1.5s, scan time= 7min.
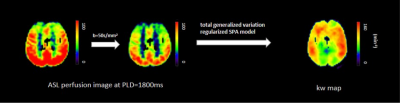
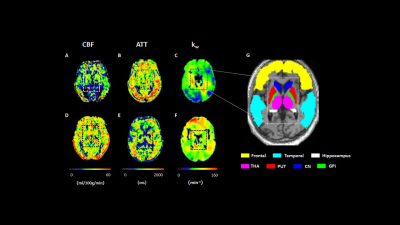
Data processing: The kw map was computed by a total-generalized-variation (TGV) regularized single-pass-approximation (SPA) model5 using the capillary fraction of the ASL signal at PLD =1800 ms (Fig. 1). Cerebral blood flow (CBF) and arterial transit times (ATT) were calculated from multi-delay pCASL protocol by a weighted-delay (WD) approach6,7 and the final CBF was the mean of the 5 individual CBFs at each PLD. The measurements of relative white matter hyperintensity (WMH) volumes were processed by the Computational Anatomy Toolbox (CAT)8 after total intracranial volume correction in SPM12 (Wellcome Trust Centre for NeuroImaging, UCL, UK). The computed kw, CBF and ATT maps were normalized into the Montreal Neurological Institute template. Individual gray and white matter masks were obtained using the standard segmentation from SPM12 with a threshold of 0.95. Other regions of interests (ROIs) were based on the AAL template: frontal and temporal lobes, hippocampus, thalamus, caudate nucleus, putamen and globus pallidus (Fig. 2G).
Data analysis: Average kw, CBF and ATT values were extracted and calculated on each ROI. After data normality analysis, normally and non-normally distributed data were compared using paired-sample t tests and Mann-Whitney U test respectively to identify the quantitative differences of kw, CBF and ATT between CARASIL and control groups. Besides, Spearmen correlation and multiple linear regression analysis were performed to evaluate the relationship between kw and other clinical parameters in CARASIL patients, including age, sex, CBF and WMH volumes. Statistical analysis was performed using the SPSS 16.0 software (SPSS, Chicago, IL). P <0.05 was considered statistically significant.
Results
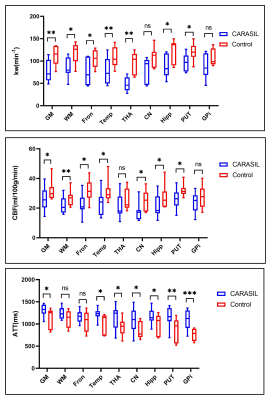
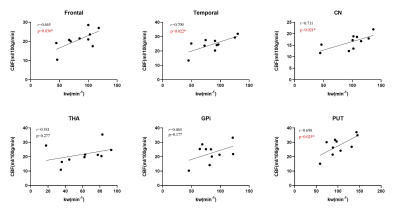
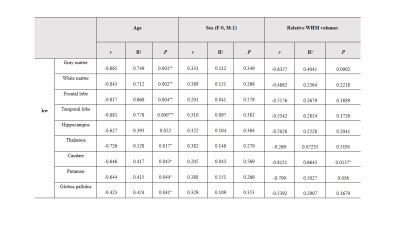
As displayed in Figs 2 and 3, significantly decreased kw of gray matter (t=3.970, p=0.007), white matter (t=3.105, p=0.021), frontal lobe (t=3.453, p=0.014), temporal lobe (t=4.092, p=0.006), thalamus (t=7.458, p=0.001), hippocampus (t=3.414, p=0.014) and putamen (t=2.550, p=0.043) were found in CARASIL patients compared with healthy controls. Besides, decreased CBF and prolonged ATT were displayed as well. Within the CARASIL group, kw was positively correlated with CBF in gray matter, frontal and temporal lobes, hippocampus, caudate nucleus and putamen (Fig. 4). Multiple linear regression analyses revealed that the kw of all ROIs except hippocampus significantly decreased with aging. Moreover, the kw of caudate nucleus was negatively associated with WMH volumes (Table 1).Discussion
Previous studies have shown that BBB impairment and cerebral hypoperfusion appear in cSVD4,9. In this study, decreased water exchange rate measured by DP-pCASL and lower CBF as well as their associations were firstly detected in patients with CARASIL. Reduced BBB water exchange has been related to impaired glymphatic function and clearance of brain waste10. Our study also found a negative correlation between kw and WMH volumes in CARASIL patients. Based on our results, kw may be a sensitive non-contrast imaging biomarker of BBB dysfunction assessment for CARASIL. Thus, further studies could be explored to investigate the underlying mechanisms of BBB dysfunction in CARASIL patients and establish the link between BBB permeability and other clinical and cognitive characteristics.Conclusion
In summary, our findings have shown significantly decreased CBF and water exchange rate across BBB in CARASIL patients, as well as the association between BBB dysfunction and clinical characteristics. The decreased BBB water exchange of CARASIL, as indicated by 3D DP-pCASL imaging, may provide a new insight on its BBB dysfunction evaluation as well as clinical assessment.Acknowledgements
This study was supported in part by Beijing Natural Science Foundation grant (L182055), Ministry of Science and Technology of China grants (2019YFC0120900, 2019YFA0707103, 2020AAA0105601), National Natural Science Foundation of China grants (81961128030, 81871350, 31730039), and the Strategic Priority Research Program of Chinese Academy of Sciences grant (XDB32010300). This study was also supported by US NIH grant R01NS114382.References
1. Cannistraro, R. J., Badi, M., Eidelman, B. H., Dickson, D. W., Middlebrooks, E. H., & 1. 1. Meschia, J. F. (2019). CNS small vessel disease: A clinical review. Neurology, 92(24), 1146–1156.
2. Ostergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, et al. Cerebral small vessel disease: capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab. 2016;36(2):302–25.
3. F.A. Nasrallah, G. Pagès, P.W. Kuchel, X. Golay, K.H. Chuang, Imaging brain deoxyglucose uptake and metabolism by glucoCEST MRI, J. Cereb. Blood Flow Metab. 33 (2013) 1270–1278
4. Wong, S. M., Jansen, J., Zhang, C. E., Hoff, E. I., Staals, J., van Oostenbrugge, R. J., & Backes, W. H. (2019). Blood-brain barrier impairment and hypoperfusion are linked in cerebral small vessel disease. Neurology, 92(15), e1669–e1677.
5. Shao, X., Ma, S. J., Casey, M., D'Orazio, L., Ringman, J. M., & Wang, D. (2019). Mapping water exchange across the blood-brain barrier using 3D diffusion-prepared arterial spin labeled perfusion MRI. Magnetic resonance in medicine, 81(5), 3065–3079.
6. Wang R, Yu S, Alger JR, et al. Multi-delay arterial spin labeling perfusion MRI inMoyamoya disease-comparison with CT perfusion imaging. Eur Radiol 2014;24:1135–44.
7. Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transitdelay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med 2012;67:1252–65.
8. Gaser C, Dahnke R, Kurth K, Luders E, Alzheimer's Disease Neuroimaging Initiative. A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. Neuroimage, in review.
9. Moreton, F. C., Cullen, B., Dickie, D. A., Lopez Gonzalez, R., Santosh, C., Delles, C., & Muir, K. W. (2021). Brain imaging factors associated with progression of subcortical hyperintensities in CADASIL over 2-year follow-up. European journal of neurology, 28(1), 220–228.
10. Gold, B.T., Shao, X., Sudduth, T.L., Jicha, G.A., Wilcock, D.M., Seago, E.R., Wang, D.J., 2021. Water exchange rate across the blood-brain barrier is associated with CSF amyloid-beta42 and cognitive performance in healthy older adults. Alzheimer's and Dementia, (Epub online).
Figures