4603
Mapping functional connectivity and brain network changes in hypertensive using resting-state fMRI1Institute of Medical Device and Imaging, Graduate Institute of Clinical Medicine, National Taiwan University, Taipei city, Taiwan, 2Department of Medical Imaging and Radiological Sciences, Graduate Institute of Artificial Intelligence, Chang Gung University, Taoyuan, Taiwan, 3Department of Medical Imaging, National Taiwan University Hospital, Taipei, Taiwan, 4School of Medicine, Chang Gung University, Taoyuan, Taiwan, 5Department of Psychiatry, Chang Gung Memorial Hospital, Chiayi, Taiwan, 6Department of Psychology, National Chung Cheng University, Chiayi, Taiwan, 7Medical Imaging Research Center, Institute for Radiological Research, Chang Gung University and Chang Gung Memorial Hospital at Linkou, Taoyuan, Taiwan
Synopsis
Although understanding of the relationship between cardiovascular disease and brain dysfunction has improved significantly over the past decades, it remains unclear whether hypertension can be a potential risk factor for cognitive impairment. This study tried to use resting-state fMRI to explore the association between hypertensive (HTN) and brain function alterations. We found significant differences in the DMN subsystems in HTN group compared with control group. We also observed higher assortativity in HTN group.
Introduction
Hypertension is a widespread disease and is considered a risk factor for cardiovascular, retinal vascular, and cerebrovascular diseases 1. Furthermore, recent findings point that hypertension can affect the brain structure and function, related to potential factors such as age, chronic hypertension, and antihypertensive medication use 2,3. However, the association between high blood pressure (BP) and cognitive brain function is complex and not yet fully understood. We aimed to investigate whether whole-brain MRI measured functional changes can be detected among individuals with/without hypertension, including the amplitude of low-frequency fluctuation (ALFF), regional homogeneity (ReHo), and graph theoretical analysis (GTA).Method
A total of 69 participants were recruited and divided into two groups, including 33 hypertensives (HTN) patients and 36 healthy controls (HC). All subjects were conducted on a 3-Tesla MRI system (Siemens Tim Trio scanner) with a standard 8-channel head coil. The resting-state functional images were acquired using a T2* weighted echo-planar imaging (EPI) sequence with the following parameter: TR/TE = 2000 ms / 30 ms, FOV = 220 mm × 220 mm, matrix size = 64 × 64, voxel size = 3.4 x 3.4 x 4, flip angle (FA) = 90°.All functional image preprocessing was performed using Statistical Parametric Mapping 12 (SPM12, Wellcome Department of Cognitive Neurology, London, UK) software. The preprocessing included the following steps. Firstly, the correction of difference in time intervals among the slices was used to eliminate time delays during imaging. Secondly, head motions were realigned by taking into account six transitional and rotational parameters. Next, images were normalized to ICBM East Asian Brain space template and then smoothed using a 6-mm full-width at half maximum (FWHM) Gaussian kernel.
After preprocessing, linear detrending and band-pass temporal filtering (0.01 to 0.12 Hz) were performed to mitigate the effects of low-frequency drifts and high-frequency physiological noise by the Resting-State fMRI Analysis tool kit (REST, Center for Cognition and Brain Disorders, Hangzhou Normal University, Zhejiang, China). Then, we used a regional homogeneity (ReHo) approach to calculate locally spontaneous synchronization of brain activity. We also conducted the amplitude of low-frequency fluctuation (ALFF) analysis to measure spontaneous neural activity. To evaluate group differences in mReHo and mfALFF, we used two-sample t-tests in SPM12, and gender, age, and education years were used as covariates to avoid the original difference. A false discovery rate (FDR)-corrected p-value of 0.05 was considered statistically significant.
In the brain networks analysis, we determined the brain networks with graph theory by using the functional connectivity toolbox (CONN, The Gabrieli Lab, McGovern Institute for Brain Research, MIT, Cambridge, MA, USA). The whole brain was divided into 90 regions of interest (ROIs) with automated anatomical labeling (AAL) template, each of which was considered a node. Then, the graph analysis toolbox (GAT, Stanford University School of Medicine, Stanford, CA, USA) was performed to analyze the topology properties of the brain network. To test for the statistical significance of the between-group differences, we manually selected the area under the curve between 0.2 and 0.5 of the density to calculate the p-value of the two-sample t-test.
Results
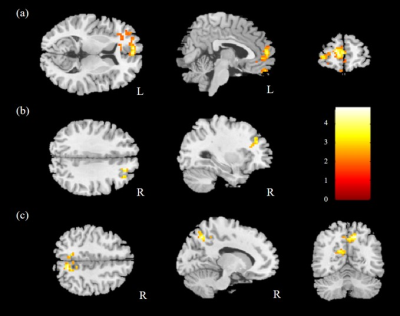
The comparison of ReHo for the two groups was performed, and significantly higher mReHo was found in the right insula, left middle occipital gyrus (MOG), and bilateral thalamus of the control group compared with the HTN group (Fig. 1). We also found significantly lower mReHo of the bilateral precuneus, superior frontal gyrus (SFG), and right middle frontal gyrus (MFG) in the control group compared with the HTN group (Fig. 2, all corrected p < 0.03).In the analysis of ALFF, we found higher mfALFF activation of the right middle occipital gyrus (MOG) and left thalamus in the control group compared with the HTN group (Fig. 3), and lower mfALFF activation of the left medial frontal gyrus, right MFG, and right precuneus in the control group compared with the HTN group (Fig. 4, all corrected p < 0.03).
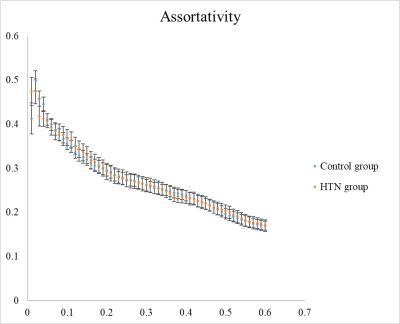
In the graph theoretical analysis as illustrated in Fig. 5, we found significantly larger assortativity in the control group compared with HTN patients (p < 0.05). Assortativity is the tendency observed in complex networks where nodes mostly connect with similar nodes.
Discussion
Our results revealed that patients with hypertension showed significantly lower mReho and mfALFF in the bilateral MOG, thalamus, and right insula, relative to the control group. It indicated these regions with lower mReho and mfALFF were associated with the vascular diseases of hypertension 4. The DMN subsystems act as a whole and interact in a dynamic equilibrium that is critical for the maintenance of normal cognition 5. Notably, HTN patients showed significantly higher mReho and mfALFF in the dorsal medial subsystem of DMN, and previous studies found a similar compensatory phenomenon in Alzheimer's disease, Parkinson’s disease, and schizophrenia 6. Related to the assortativity of the brain network, this compensation can be seen as a process of brain reconstruction and brain functional remodeling due to its plasticity after damage to original neural networks 7.Conclusion
We concluded that the regional brain function of the hypertensive patients was different from the healthy participants. The findings from the present study indicated that hypertension might potentially damage the visual function and further weaken cognitive control.Acknowledgements
This study was supported by grants CMRPG6I0051 from Chang Gung Memorial Hospital, Chiayi, Taiwan.References
1. Roditi, G., MR in hypertension. J Magn Reson Imaging, 2011. 34(5): p. 989-1006.
2. Swan, G.E., et al., Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology, 1998. 51(4): p. 986-93.
3. Gottesman, R.F., et al., Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol, 2014. 71(10): p. 1218-27.
4. Gao, Q., et al., Assessment of cerebral low-frequency oscillations in patients with retinal vein occlusion: a preliminary functional MRI study. Acta Radiol, 2020. 61(6): p. 813-820.
5. Haight, T.J., et al., Vascular risk factors, cerebrovascular reactivity, and the default-mode brain network. Neuroimage, 2015. 115: p. 7-16.
6. Son, S.J., et al., Effect of hypertension on the resting-state functional connectivity in patients with Alzheimer's disease (AD). Arch Gerontol Geriatr, 2015. 60(1): p. 210-6.
7. Gu, Y., et al., Characteristic changes in the default mode network in hypertensive patients with cognitive impairment. Hypertens Res, 2019. 42(4): p. 530-540.
Figures
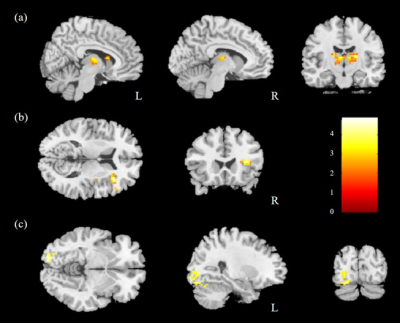
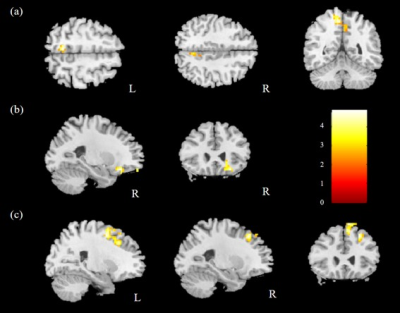
Fig. 2. Lower mReHo was observed in the (a) bilateral precuneus, (b) right MFG, and (c) bilateral SFG in the control group compared with the HTN group with corrected p<0.03 and cluster size>100.
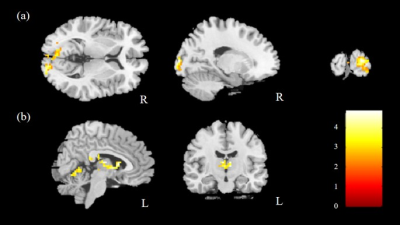
Fig. 3. Higher mfALFF was found in the (a) right MOG and (b) left thalamus in the control group compared with the HTN group with corrected p<0.03 and cluster size>100.