4602
Assessment of cerebrovascular related tissue pH in patients with moyamoya disease: an amide proton transfer weighted imaging study1Huaxi MR Research Center (HMRRC), Functional and Molecular Imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Sichuan University, Chengdu, China, 2Research Unit of Psychoradiology, Chinese Academy of Medical Sciences, Chengdu, China, 3Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, China, 4Philips Healthcare, Xi'an, China
Synopsis
The amide proton transfer weighted (APTw) imaging was used to investigate the collateral circulation change and pH alteration in patients with moyamoya disease (MMD). Thirteen patients underwent computed tomography perfusion (CTP), digital subtraction angiography (DSA), and APTw imaging. We analyzed the difference in APTw values between the cerebral and cerebellar hemispheres. Then subgroup analysis was conducted according to the stage of preinfarction period based on CTP. The results revealed that APTw values were significantly lower in cerebral hemispheres than those in cerebellar hemispheres. However, no significant difference in APTw values among patients with different stages of preinfarction period was found.
Introduction
Amide proton transfer weighted (APTw) imaging is a non-invasive magnetic resonance imaging (MRI) technique that is sensitive to the exchange of amide protons and free protons. 1 Due to the increased concentration of free proteins in tumor tissues which can contribute to APTw signal (APTw value, quantified with the magnetization-transfer-ratio asymmetry at 3.5 ppm), APTw imaging has been widely used in oncologic diagnosis, especially in the brain. It's worth noting that the APTw signal is also sensitive to the pH of tissue microenvironment. Previous studies have shown that tissue acidification during anaerobic metabolism can be detected by APTw imaging in patients with ischemic stroke. 2, 3 Moyamoya disease (MMD) is a specific chronic cerebrovascular occlusive disease characterized by long-standing and progressive occlusion of large intracranial arteries.4 Due to its smoke-liked vessels, the hemodynamic characteristics of MMD change differently. To our knowledge, no previous researches evaluated the pH of cerebral tissue in patients with MMD by using APTw imaging. Therefore, this pilot study aimed to investigate the collateral circulation change and pH alteration in MMD.Materials and Methods
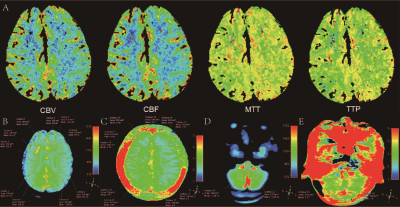
Thirteen patients (46±14 years; range 17-64 years) with MMD were enrolled from West China Hospital. All patients underwent computed tomography perfusion (CTP) and digital subtraction angiography (DSA) examination to confirm the diagnosis of MMD before MRI scanning. We further divided the 13 patients into four subgroups according to the stage of preinfarction period based on CTP. 5 Then all of them underwent MR imaging on a 3T scanner (Ingenia Elition, Philips Healthcare, the Netherlands) using a 32-channel head coil. The sequences and parameters including 3D-T1-TFE with repetition time / echo time (TR/TE) = 1,730/2.19 ms; slice thickness = 1.0 mm; field of view (FOV) = 256*256 mm2; number of slices = 176; flip angle = 9 degrees; voxel = 1.0*1.0*1.0 mm3. The APTw imaging was performed with 3D TSE-DIXON sequence with TR/TE = 6,120/7.8 ms; slice thickness = 6.0 mm; FOV = 230*180 mm2; flip angle = 90 degrees; voxel = 1.8*1.8*6.0 mm3; saturation power = 2 μT; duration = 2,000 ms. Before region of interests (ROIs) drawing, the APTw images were automatically co-registered to the structural 3D-T1-TFE images by performing a rigid transformation of the datasets. The placement of ROIs (90~110 pixels each) was as follows: according to the site of impaired cerebral perfusion on CTP, ROIs were firstly drawn in cerebral hemispheres in the magnitude imaging of APTw, which had good resolution. ROIs would be placed in multiple slices if cerebral perfusion was extensively impaired. The ROIs were then copied from the magnitude to the APTw imaging to ensure that their locations were the same. To compare with normal tissue, a reference ROI was placed at normal-appearing white matter (NAWM) in the ipsilateral cerebellum region. Two senior radiologists (both with 7 or more years of experience) manually drew ROIs independently. The intraclass correlation coefficient (ICC) was performed to evaluate the inter-observer consistency of the APTw values. After obtaining the minimum (APTw_min) and mean (APTw_mean) of APTw values, the Student t-test was performed to analyze the difference between the cerebral and the cerebellar hemispheres. And ANOVA tests were employed in the subgroup analysis.Results
The variability of the two radiologists was pretty good (ICC value > 0.9). Table 1 shows the detailed demographic and clinical information of 13 patients with MMD. Figure 1 illuminates a typical example of MMD patient and related ROIs placement. Compared with the cerebellar hemisphere, both APTw_min (0.29±0.37 vs. 1.22±0.31, P <0.001) and APTw_mean (0.62±0.31 vs. 1.22±0.31, P <0.001) in cerebra were significantly lower (Figure 2). However, no significant difference in APTw_min or APTw_mean among patients with different stages of preinfarction period were found (all P >0.05) (Table 2).Discussion
The most important pathophysiology of MMD is the progressive stenosis and/or occlusion of the terminal portions of the bilateral internal carotid arteries.4 In most cases, MMD affects the vessels of the anterior circulation of the brain and rarely implicates the vertebrobasilar system.6 Our results demonstrated that APTw values in the cerebral hemisphere were significantly lower than those in cerebellar hemispheres, indicating that the internal environment of cerebral tissue of patients with MMD was acidic. Therefore, this study validated the above phenomenon from the perspective of pH metabolism of brain tissue in MMD. Surprisingly, no significant difference in APTw values among patients with different stages of preinfarction period was found. Theoretically, the higher the preinfarction period stage, the more serious the impaired brain perfusion, that is to say, the more obvious the acidosis of brain tissue. In addition, previous studies suggested patients with MMD with low preinfarction period stages (I and II) had a better prognosis after surgical treatment.5 We suspected that one of the possible reasons was the small sample size in our study. In the future, multi-center studies with a large sample size should be carried out to confirm our results. Furthermore, APTw imaging might be used to estimate the efficacy of bypass surgery for MMD.Conclusion
APT-based pH evaluation can effectively reflect the alteration of tissue microenvironment caused by impaired hemodynamics in patients with MMD. And it’s a promising method that may provide valuable neuroimaging biomarkers for preoperative assessment and surgery planning.Acknowledgements
No acknowledgement found.References
1. Jones CK, Schlosser MJ, Zijl PC, et al. Amide proton transfer imaging of human brain tumors at 3T. Magnetic Resonance in Medicine, 2006, 56(3): 585-592.
2. Song G, Li C, Luo X, et al. Evolution of Cerebral Ischemia Assessed by Amide Proton Transfer-Weighted MRI. Frontiers in neurology, 2017, 8: 67.
3. Lee SF, Harston G, Mehndiratta A, et al. Clinical translation of amide proton transfer (APT) MRI for ischemic stroke: a systematic review (2003-2020). Quant Imaging Med Surg, 2021, 11(8): 3797-3811.
4. Parray T, Martin TW, Siddiqui S. Moyamoya disease: a review of the disease and anesthetic management. J Neurosurg Anesthesiol, 2011, 23(2):100-109.
5. Yin H, Liu X, Zhang D, et al. A novel staging system to evaluate cerebral hypoperfusion in patients with moyamoya disease. Stroke, 2018, 49: 2837-2843.
6. Tan C, Niu H, Duan R, et al. Abnormal Embryonic Development of Cerebral Arteries as a Potential Cause of Moyamoya Disease. World Neurosurgery, 2019, 129: e224-e232.
Figures