4599
Deep Learning regularized SPIRiT reconstruction accelerates joint intracranial and carotid vessel wall imaging into 3.5 minutes1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Shenzhen, China, 2Research Center for Medical AI, Shenzhen Institutes of Advanced Technology, Shenzhen, China, 3Northern Jiangsu People's Hospital, Yangzhou, China
Synopsis
Deep learning regularized SPIRiT reconstruction is developed by unrolling the conventional L1-SPIRiT optimization solved by the projection onto convex sets (POCS) iteration into a multi-layer convolutional neural network. The learnable network regularization with 3D convolution improved the reconstruction accuracy and efficiency compared with the iterative L1-SPIRiT with 2D sparsity regularization in a fixed transform domain. The simplicity of POCS iteration also benefits the design complexity of the DL-SPIRiT network. The proposed DL-SPIRiT could accelerate the joint intracranial and carotid vessel wall imaging of isotropic 0.6 mm resolution by 8-fold, leading to a scan time of only 3.5 minutes.
Introduction
Combined Compressed Sensing and Parallel Imaging (CSPI) 1 has been utilized recently to reduce the scan time of high-resolution (isotropic 0.5-0.6 mm) MR vessel wall imaging (VWI) from 9-10 minutes 2,3 to 5-6 minutes using 5-fold acceleration 4,5. However, CSPI accelerated VWI faces the following challenges in clinical practice: 1. needs empirically adjusting the sparsity regularization weight for different subjects 4; 2. needs long reconstruction time due to the iterative solver and huge VWI data size 5, and 3. is susceptible to the blurring of image details especially at higher acceleration factor 4,5. This work proposed deep learning (DL) reconstruction for 8-fold accelerated VWI by unrolling 6 the L1-SPIRiT optimization solved by the POCS (projection onto convex sets) algorithm 1 to a learnable convolution neural network (CNN). The above limitations of iterative CSPI reconstruction could be alleviated, leading to high-fidelity joint intracranial and carotid VWI of isotropic 0.6 mm resolution in 3.5 minutes.Methods
The whole 3D reconstruction is decomposed to multiple 2D reconstruction tasks after 1D inverse Fourier Transform (FT) along the fully sampled readout. The POCS iteration solving the L1-SPIRiT optimization is unrolled to a multi-layer CNN. Each layer computes the 2D SPIRiT self-consistency convolution and the acquisition data consistency (DC) in k-space as each POCS iteration. A CNN block replaces the original slice-by-slice 2D image sparsity regularization with 3D convolution to exploit the inter- and intra-slice correlation, thanks to the high isotropic resolution used in VWI. Inputs to the proposed DL-SPIRiT network are undersampled k-space of multiple adjacent slices and corresponding SPIRiT kernels estimated from the central autocalibration scans (ACS), and outputs are reconstructed multi-slice magnitude images (coil combination by sum-of-squares). The structure of the proposed DL-SPIRiT network is illustrated in Figure 1.Institutional Review Board approved in-vivo experiments with informed consent obtained from all volunteers. All VWI scans were performed on a 3T scanner (uMR 790, United Imaging Healthcare, China) with a 32-channel head and neck coil. Common imaging parameters included: T1 weighted MATRIX (Modulated flip Angle Technique in Refocused Imaging with eXtended echo train) sequence with non-selective excitation and sagittal imaging orientation, FOV = 220 (RO) x 200 (PE1) x 154-172 (PE2) covering both intracranial and carotid arteries, resolution = isotropic 0.6 mm, TR/TE = 800/13.6 ms, echo train length = 46, bandwidth = 500 Hz/pixel. Fully sampled VWI scan were performed on eight healthy volunteers (mean age 26 years, five females) with scan durations between 18 and 22 minutes. Five fully sampled datasets were retrospectively undersampled by 8-fold variable-density Poisson-disc undersampling (vdPDS) for training, while the other three datasets were used for retrospective testing. VWI scans accelerated prospectively by 8-fold vdPDS were also performed on another two healthy volunteers (26 and 56 years old, male) with scan durations of 3.2 and 3.5 minutes, respectively. The DL-SPIRiT network was implemented in TensorFlow 2.2 and trained on an Nvidia Quadro RTX 8000 GPU with 48 GB memory.
Results
Figure 2 demonstrates the ablation study on analyzing the effects of SPIRiT model and 3D CNN convolution on DL-SPIRiT reconstruction quality using a fully sampled VWI data and simulated 8-fold vdPDS acceleration. The SPIRiT model could effectively suppress the aliasing induced by 8-fold undersampling, while the 3D convolution gave a more accurate reconstruction of image details than 2D convolution.Figure 3 investigates the effects of unrolled CNN layer number on DL-SPIRiT reconstruction accuracy. Ten to twelve layers could better balance reconstruction accuracy and efficiency, leading to higher computational performance than the conventional POCS solver which usually requires 30-40 iterations, due to the reduced number of computing the time-consuming coil-by-coil SPIRiT convolution.
Figure 4 shows the superior reconstruction quality of DL-SPIRiT compared with the state-of-the-art DL-ESPIRiT 7,8 and the conventional L1-SPIRiT on an 8-fold accelerated VWI data. Compared with DL-ESPIRiT, which needs estimating 3D coil sensitivity maps (CSM) using amounts of signal value decomposition (SVD), DL-SPIRiT computes kernel calibration more efficiently by simple least squares minimization and could reconstruct images without artifacts induced by phase singularities in the CSM.
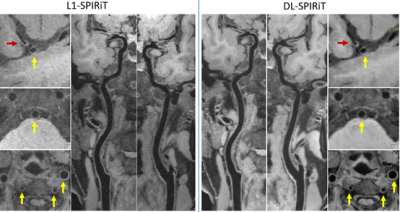
Figure 5 illustrates the superior noise suppression and vessel wall depiction quality of DL-SPIRiT than L1-SPIRiT on a prospective 8-fold accelerated VWI data acquired in 3.5 minutes.
Discussion
This work developed DL-SPIRiT reconstruction for 8-fold accelerated VWI of isotropic 0.6 mm resolution by unrolling the conventional L1-SPIRiT optimization solved by the POCS algorithm into a multi-layer CNN. The learnable network regularization with 3D convolution improved reconstruction accuracy and efficiency than iterative L1-SPIRiT with 2D sparsity regularization in a fixed transform domain. The simplicity of POCS iteration also benefits the design complexity of the proposed DL-SPIRiT network. Further work will focus on investigating the generality and robustness of applying the DL-SPIRiT accelerated VWI in clinical practice.Acknowledgements
This work is supported by the State Key Program of National Natural Science Foundation of China (Grant No. 81830056) and the National Natural Science Foundation of China (Grant No. 81801691).References
1. Murphy M, Alley M, Demmel J, et al. Fast l1-SPIRiT Compressed Sensing Parallel Imaging MRI: Scalable Parallel Implementation and Clinically Feasible Runtime. IEEE Trans. Medical Imaging 2012, 31:1250-1262.
2. Zhang L, Zhang N, Wu J, et al. High resolution simultaneous imaging of intracranial and extracranial arterial wall with improved cerebrospinal fluid suppression. Magn Reson Imaging 2017; 44:65-71.
3. Fan Z, Yang Q, Deng Z et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D Turbo Spin Echo. Magn Reson Med 2017; 77:1142-1150.
4. Jia S, Zhang L, Ren L, et al. Joint Intracranial and Carotid Vessel Wall Imaging in 5 minutes using Compressed Sensing accelerated DANTE-SPACE. Eur Radiol 2020, 30:119-127.
5. Zhu C, Tian B, Chen L, et al. Accelerated whole-brain intracranial vessel wall imaging using black-blood fast spin-echo with compressed sensing (CS-SPACE). Magn Reson Mater Phy 2018; 31, 457–467.
6. Monga V, Li YL, Eldar YC. Algorithm Unrolling: Interpretable, efficient deep learning for signal and image processing. IEEE Signal Processing Magazine 2021; 38:18-44.
7. Sandino CM, Lai P, Vasanawala SS, et al. Accelerating cardiac cine MRI using a deep learning-based ESPIRiT reconstruction. Magn Reson Med 2021; 85:152-167.
8. Hammernik K, Schlemper J, Qin C, et al. Systematic evaluation of iterative deep neural networks for fast parallel MRI reconstruction with sensitivity-weighted coil combination. Magn Reson Med 2021; 86:1859-1872.
Figures