4596
Accuracy of Characterizing Carotid Vulnerable Atherosclerotic Plaque by 3D MR Vessel Wall Imaging: A Histological Validation Study1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2Department of Neurosurgery, Peking University Third Hospital, Beijing, China, 3China National Clinical Research Center for Neurological Disease, Beijing Tiantan Hospital, Capital Medical University, Beijing, China, 4Department of Radiology, Peking University Third Hospital, Beijing, China, 5Department of Radiology, Sun Yat-Sen Memorial hospital, Sun Yat-Sen University, Guangzhou, China, 6Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Capital Medical University, Beijing, China, 7Department of Radiology, Beijing Geriatric Hospital, Beijing, China, 8Thorough Images, Beijing, China, 9Philips Healthcare, Shanghai, China
Synopsis
This study investigated the accuracy of 3D multi-contrast MR vessel wall imaging (VWI) in characterizing carotid vulnerable plaque compositions including lipid-rich necrotic core (LRNC), intraplaque hemorrhage (IPH) and calcification (CA) validated by histology. Good agreements were found between MR and histology in identifying LRNC (κ=0.67), IPH (κ=0.66), and CA (κ=0.62) after excluding histological sections with the plaque components <1.77 mm2. Moderate agreements were reached in quantifying plaque compositions with r values ranged from 0.46 to 0.61 (LRNC: r=0.52; IPH: r=0.6; CA: r=0.46). Our study demonstrated that 3D multi-contrast MR VWI is capable of accurately characterizing carotid vulnerable atherosclerotic plaques.
Introduction
Stroke is a major cause of disability and death worldwide1. Disruption of carotid vulnerable atherosclerotic plaques is an important etiology for ischemic cerebrovascular events2,3. Vulnerable plaque is characterized by presence of plaque compositional features including large lipid-rich necrotic core (LRNC), intraplaque hemorrhage (IPH), surface or multiple calcifications (CA), and rapture of fibrous cap4,5. Therefore, accurate evaluation of carotid plaque compositional features is critical for the prevention of cerebrovascular events. The 2D multi-contrast magnetic resonance (MR) vessel wall imaging (VWI) has been demonstrated to be capable of identifying and quantifying the plaque compositions validated by histology. Due to smaller longitudinal coverage, longer scan time, and lower inter-slice resolution of 2D MR VWI, 3D MR VWI techniques such as T1- and T2-VISTA/SPACE/CUBE sequences have been largely applied to clinical settings for assessing carotid vulnerable plaque characterization. However, the accuracy of 3D MR VWI in characterizing carotid vulnerable plaque features has not been validated by histology. This study sought to histologically validate the accuracy of 3D MR VWI techniques in evaluating carotid vulnerable plaque compositional features.Materials and Methods
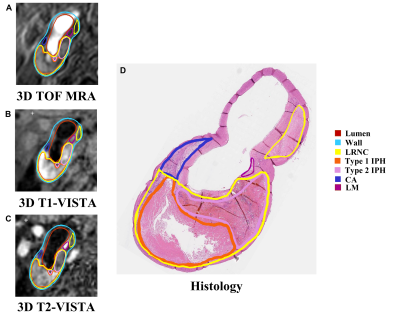
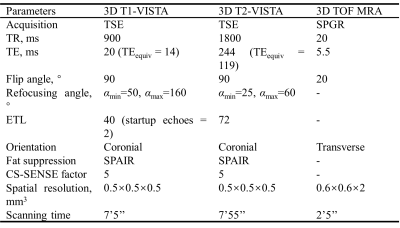
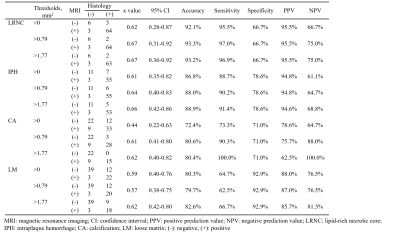
Study sample: A total of 21 patients (mean age: 64.4±7.2 years; 21 males) with carotid atherosclerotic disease scheduled to carotid endarterectomy (CEA) (symptomatic 50-70% stenosis or >70% stenosis) were recruited.MR imaging: All patients underwent 3D multi-contrast MR VWI for carotid arteries on a whole-body 3.0 T MR scanner (Ingenia CX, Philips Healthcare, Best, The Netherlands) with a dedicated 8-channel carotid coil and 32-channel head coil within one week before CEA. The imaging protocol includes 3D T1-VISTA, 3D T2-VISTA, and 3D TOF MRA sequences and the imaging parameters were detailed in Table 1. The 3D T1-VISTA and 3D T2-VISTA were reconstructed into cross-sectional images with 2 mm slice thickness to register with the 3D TOF MRA. The specimens of carotid plaques obtained from a standard CEA procedure were sectioned (10 m) every 0.5 mm and stained with hematoxylin and eosin.
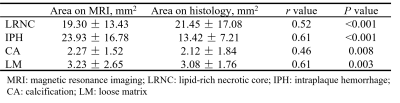
Histology and MR data analysis: Histological sections were analyzed by a histologist with >5 years’ experience in pathology of atherosclerosis blinded to MR images. The LRNC, type 1 IPH, type 2 IPH, CA, and loose matrix (LM) were evaluated using Thorough Wisdom software (Thorough Images, China) and published criteria6. The histological sections were matched with the 3D multi-contrast VWI images. All matched MR VWI images were analyzed by 2 radiologists with >3 years’ experience in vascular imaging with consensus utilizing VesselExplorer 2.0 software (TSImaging Healthcare, Beijing, China) blinded to histological results. The presence and areas of LRNC, type 1 IPH, type 2 IPH, CA and LM were evaluated on MR images using the published criteria7.
Statistical analysis: Cohen’s kappa (κ) analysis was used to determine the agreement between 3D multi-contrast MR VWI and histological sections in identifying carotid plaque compositions after exclusion of histological plaque compositions smaller than certain thresholds which was 0 mm2, 0.79 mm2 and 1.77 mm2, respectively3. The type 1 and type 2 IPH were integrally evaluated. The accuracy, specificity, sensitivity, positive prediction value (PPV), and negative prediction value (NPV) of 3D multi-contrast MR VWI in identifying carotid plaque compositions were calculated. The agreement of the area for each plaque composition measured by MR VWI and histology was determined using Spearman’s rho coefficients and Bland Altman plots. For better matching with histology, the area of plaque composition measured by MR VWI was reduced by 7.8% due to the shrinkage of histological processing3. The study protocol was approved by the local institutional review board and all participants provided written consent form.
Results
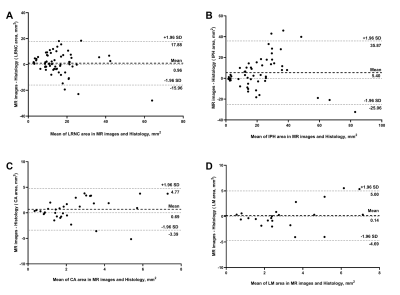
Of the 270 histological sections from 21 patients, 76 were successfully matched with carotid MR images. The κ values, accuracy, sensitivity, specificity, PPV and NPV of 3D multi-contrast MR VWI in detecting plaque compositions ranged from 0.44 to 0.67, 72.4% to 93.3%, 62.5% to 100%, 66.7% to 92.9%, 62.5% to 95.5%, and 61.1% to 100%, respectively (Table 2). After excluding histological sections where plaque components were under 1.77 mm2, the highest κ value (LRNC: 0.67, IPH: 0.66; CA: 0.62, LM: 0.62) was reached and good agreement of each component between MR and histology was achieved (Figure 1, Table 2). Moderate agreements were reached in quantification of all components with r value ranged from 0.46 to 0.61 (Table 3). The Bland-Altman plots indicate that the bias in measuring plaque compositions ranged from 0.14 mm2 to 5.40 mm2 (Figure 2).Discussion and conclusion
In the present study, moderate to good agreements were found between 3D MR VWI and histology in identifying and quantifying carotid vulnerable plaque features. Our 3D MR VWI showed better performance in identifying (κ value with histology: 0.62 vs. 0.54) and quantifying IPH (r value with histology: 0.61 vs. 0.50) than previous 2D MR VWI reports8, which benefited from its higher in-plane (0.5 mm×0.5 mm vs. 0.6 mm×0.6 mm) and inter-plane resolution (0.5 mm vs. 2 mm). Similar to our results, Saam et al7 found that there were good agreement and moderate to strong correlation in identifying (LRNC: κ=0.73; IPH: κ=0.71) and quantifying LRNC (r=0.75) and IPH (r=0.66) whose areas were > 2 mm2 between MR imaging and histology, respectively. In conclusion, 3D multi-contrast MR VWI enables accurately characterizing carotid vulnerable atherosclerotic plaques validated by histology.Acknowledgements
No acknowledgement found.References
1. Hurd MD, Goel I, Sakai Y, Teramura Y. Current status of ischemic stroke treatment: From thrombolysis to potential regenerative medicine. Regen Ther. 2021;18:408-417.
2. Zhao H, Zhao X, Liu X, Cao Y, Hippe DS, Sun J, Li F, Xu J, Yuan C. Association of carotid atherosclerotic plaque features with acute ischemic stroke: a magnetic resonance imaging study. Eur J Radiol. 2013;82(9):e465-70.
3. Li D, Qiao H, Han Y, Han H, Yang D, Cao J, Xu H, Wang T, Wang Y, Shen J, Zhao X. Histological validation of simultaneous non-contrast angiography and intraplaque hemorrhage imaging (SNAP) for characterizing carotid intraplaque hemorrhage. Eur Radiol. 2021;31(5):3106-3115.
4. Insull W Jr. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122(1 Suppl): S3-S14.
5. Lusby RJ, Ferrell LD, Ehrenfeld WK, Stoney RJ, Wylie EJ (1982) Carotid plaque hemorrhage. its role in production of cerebral ischemia. Arch Surg 117:1479–1488.
6. Stary HC. Natural history and histological classification of atherosclerotic lesions: an update. Arterioscler Thromb Vasc Biol. 2000;20:1177–1178.
7. Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005 ;25(1):234-9.
8. Ota H, Yarnykh VL, Ferguson MS, Underhill HR, Demarco JK, Zhu DC, Oikawa M, Dong L, Zhao X, Collar A, Hatsukami TS, Yuan C. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology. 2010;254(2):551-63.
Figures