4594
MRI Radiomics approach for Identification of high-risk intracranial plaque basing on machine learning
Haining Wei1, Fang Wu2, Jie Lu2, and Rui Li1
1Tsinghua University, Beijing, China, 2Xuanwu Hospital, Capital Medical University, Beijing, China
1Tsinghua University, Beijing, China, 2Xuanwu Hospital, Capital Medical University, Beijing, China
Synopsis
Intracranial atherosclerotic disease (ICAD) is one of the main causes of ischemic stroke. Increasing evidence supports that vulnerable plaque is correlated with risk for stroke, which reveals the importance of intracranial plaque risk identification. Radiomics is an automated and repeatable approach for extracting massive features for medical imaging. However, few articles have focused on radiomic-based studies of intracranial plaque of basilar artery and middle cerebral artery. In this study, we propose to build a high-risk intracranial plaque model using radiomics features and machine learning to differentiate symptomatic plaque from asymptomatic plaque, which is helpful in guiding clinical management.
INTRODUCTION
Intracranial atherosclerotic disease (ICAD) is one of the main causes of ischemic stroke, which is the leading cause of death and disability worldwide [1]. As a systemic disease, risk factors for symptomatic and asymptomatic ICAD are complex. Increasing evidence supports that vulnerable plaque is correlated with risk for stroke, which reveals the importance of intracranial plaque risk identification. Magnetic resonance imaging (MRI) has been established as a reliable and non-invasive clinical method for plaque characterization. Due to the MRI can provide a variety of vessel wall information, experts need to evaluate quantitatively and qualitatively. Despite of these visual information, some additional features based on data statistics are also benefit for building risk prediction model. Radiomics is an automated and repeatable approach for extracting massive features for medical imaging. However, few articles have focused on radiomic-based studies of intracranial plaque of basilar artery and middle cerebral artery. In this study, we propose to build a high-risk intracranial plaque model using radiomics features and machine learning to differentiate symptomatic plaque from asymptomatic plaque, which is helpful in guiding clinical management.METHODS
- Patient inclusion criteria
- MRI acquisition and segmentation
- Model development
RESULTS
We use a 5-fold cross validation to evaluate the performance of the machine learning models and also calculate the average receiver operating characteristic (ROC) analysis and the area under the ROC curve (AUC). The cross-validation results and the mean ROC curve are shown in Figure 4. As shown in results, when combining all the radiomic features from precontrast T1 and postcontrast T1 images, the AUC value improved. We also test all features on multiple machine learning methods. The SVM method shows the best performance with mean AUC of 0.88 compared with other familiar regression models.DISCUSSION and CONCLUSION
In this study, we adopt radiomics to acquire richer information and focus on high-risk intracranial plaque, which is rarely investigated by radiomics analysis. The model consists of 26 quantitative features after dimension reduction and achieves excellent diagnostic performance, which are not perceived by radiologists. However, the relatively small number of patients and single medical center are still inconvenienced the performance and stability of method. Our future work will focus on combining model of traditional qualitative indicators, radiomics features and deep learning based information from images.Acknowledgements
No acknowledgement found.References
[1] Brent Flusty, Adam de Havenon, Shyam Prabhakaran, David S. Liebeskind, Shadi Yaghi, Intracranial Atherosclerosis Treatment. Stroke. 2020;51:e49–e53
[2] van Griethuysen, J. J. M., Fedorov, A., Parmar, C., Hosny, A., Aucoin, N., Narayan, V., Beets-Tan, R. G. H., Fillion-Robin, J. C., Pieper, S., Aerts, H. J. W. L. (2017). Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Research, 77(21), e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Figures
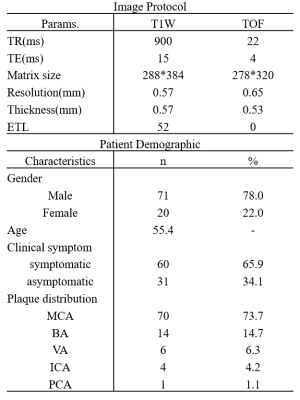
Table 1. Image protocol settings and patient demographic data. A few patients had more than one intracranial plaque. Middle cerebral artery(MCA), Basilar artery(BA), Vertebral artery(VA), Internal carotid artery(ICA), Posterior carotid artery(PCA).
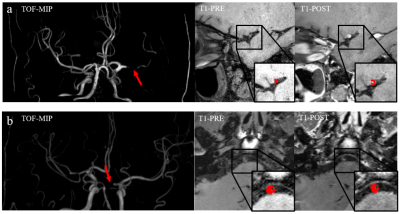
Figure 1. MR segmentation examples of (a) symptomatic group and (b) asymptomatic group. Each group displays the MIP, precontrast T1 and postcontrast T1 images. The red arrows demonstrate stenosis and the red mask extracted from the ROIs corresponding to the images
Figure 2. Correlation heat map among the selected features in precontrast T1 and postcontrast T1
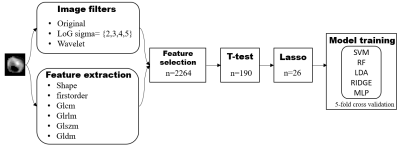
Figure 3. Flowchart of study process
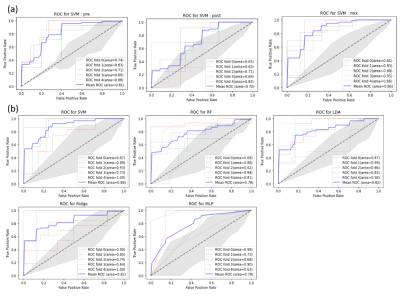
Figure 4. The ROC curves to different machine learning method. Each subgraph shows the 5-fold cross-validation results and the mean ROC curve. The area stands for AUC. The gray area is true positive rate distribution. Group (a) and (b) are trained under different dataset split. (a) results of precontrast T1 and postcontrast T1 features via SVM. (b) results of all features via multi-method.
DOI: https://doi.org/10.58530/2022/4594