4591
Altered Spontaneous Brain Activity Related to Neurologic Dysfunction in Patients with Cerebral Small Vessel Disease: A resting-state study1Shandong Provincial Hospital, Jinan, China, 2Key Laboratory of Cognition and Personality (Ministry of Education), Chongqing, China, 3Southwest University, Chongqing, China, 4Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Synopsis
Cerebral small vessel disease (CSVD) patients can present structural and functional abnormalities. We divided 102 subjects into three groups and obtained information about altered spontaneous brain activity using amplitude of low-frequency fluctuation (ALFF), fractional ALFF (fALFF) and regional homogeneity (ReHo) methods based on resting-state functional MRI. Compared with controls and the CSVD without cerebral microbleeds (CSVD-n) group, the CSVD with cerebral microbleeds (CSVD-c) group showed significantly increased ALFF/ReHo values and decreased fALFF values in some brain regions, which are correlated to abnormal clinical characteristics. These results expounded the underlying neurophysiological mechanisms in CSVD patients.
Methods In this study, we recruited 24 CSVD patients with CMBs (CSVD-c), 42 CSVD patients without CMBs (CSVD-n) and 36 healthy controls from outpatient clinics in Shandong Provincial Hospital affiliated with Shandong First Medical University between September 2018 and June 2019. All subjects underwent 3-T MRI, including three-dimensional T1-weighted (T1W), blood oxygen level-dependent (BOLD) and susceptibility-weighted imaging (SWI). Anatomic structures were segmented, ALFF/fALFF values were calculated, and ReHo maps were generated. Further statistical analysis was applied to study the difference in ALFF/fALFF/ReHo among the three groups and the association between ALFF/fALFF/ReHo changes in different brain regions and clinical characteristics.
Results Twenty-four CSVD-c patients (age: 67.54±6.00 years, 10 females), 42 CSVD-n patients (age: 66.33±5.25 years, 22 females) and 36 healthy subjects (age: 64.14±8.57 years, 19 females) were evaluated. The demographic and clinical characteristics of each group are summarized (Figure 1). Compared with controls, the CSVD-c group showed significantly increased ALFF values in the right insula, putamen and left precuneus; decreased fALFF values in the right precentral gyrus and postcentral gyrus; and increased ReHo values in the left precuneus, fusiform gyrus, right supplementary motor area, and superior frontal gyrus (Figure 2/3). No significant difference was found between the CSVD-n group and the control group. Notably, the mean ALFF values of the right insula and putamen were not only significantly related to all clinical parameters but also demonstrated the best performance in ROC curve analysis (Figure 4/5).
Discussion Only CSVD-c patients had apparent increased/decreased ALFF/fALFF/ReHo in different brain regions compared with controls and CSVD-n patients. A previous study suggested that leakage of the blood-brain barrier (BBB) is often considered to be the original mechanism of CSVD, which can lead to immune cell infiltration and inflammation and result in a variety of pathological processes.2 As one of the manifestations of CSVD, there is a close relationship between microbleeding and vascular inflammation markers. The greater burden of CMBs is often accompanied by increased markers of vascular inflammation/endothelial dysfunction and systemic inflammation.3 All inflammatory markers were at higher levels in CMBs patients, and compared with CSVD-n patients, CSVD-c patients had more severe pathological changes.4 This also explains why the CSVD-c group showed elevated ALFF values compared with those of the CSVD-n group, and the changed area was similar to the changed area of the controls but slightly less. Therefore, compared with controls, only the ALFF/fALFF/ReHo values of CSVD-c patients changed significantly. CSVD-c patients showed increased ReHo in some regions compared with that of CSVD-n patients.
Compared with controls, CSVD-c patients showed increased ALFF in some regions that link with various neural pathways and networks to influence cognitive control processes, sensorimotor processing and affective processes, executive function in the CSVD-c group compared with controls.5-8 Significantly decreased fALFF values were found in the right precentral gyrus and postcentral gyrus in CSVD-c patients compared with controls. The postcentral gyrus serves as a key region in the somatosensory network, participates in daily activities and governs the learning of early motor skills acquisition.9,10 Therefore, CSVD-c patients may experience reduced motor learning ability compared to the control group. Brain regions with increased ReHo values are associated with facial recognition, resting-state regulation, working memory compared with controls/CSVD-n patients.11-17 Therefore, changes in the ALFF/fALFF/ReHo value of these areas impair related cognitive function parameters.
Conclusion In the current study, we investigated the possible pathogenesis of CSVD by analyzing resting-state spontaneous brain activity based on ALFF, fALFF and ReHo values in CSVD patients. The results suggested that abnormal changes in spontaneous brain activity in the default mode Network, somatosensory network, sensorimotor network, and frontoparietal network may explain the changes in clinical parameters in CSVD patients, especially in CSVD-c patients. The correlation between altered spontaneous neuronal activity and clinical parameters provides early useful diagnostic biomarkers for CSVD.
Acknowledgements
We thank all of the volunteers and patients for their participation in our study.References
1. Shi Y, Wardlaw J M. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. 2016;1:83-92.2. Cuadrado-Godia E, Dwivedi P, Sharma S, et al. Cerebral Small Vessel Disease: A Review Focusing on Pathophysiology, Biomarkers, and Machine Learning Strategies. J Stroke. 2018;20:302-320.
3. Low A, Mak E, Rowe J B, et al. Inflammation and cerebral small vessel disease: A systematic review. Ageing Res Rev. 2019;53:100916.
4. Miwa K, Tanaka M, Okazaki S, et al. Relations of blood inflammatory marker levels with cerebral microbleeds. Stroke. 2011;42:3202-3206.
5. Chang L J, Yarkoni T, Khaw M W, et al. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739-749.
6. Deen B, Pitskel N B, Pelphrey K A. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498-1506.
7. Uddin L Q, Kinnison J, Pessoa L, et al. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 2014;26:16-27.
8. Kokubo K, Suzuki K, Hattori N, et al. Executive Dysfunction in Patients with Putaminal Hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:1978-1985.
9. Fu J, Chen X, Gu Y, et al. Functional connectivity impairment of postcentral gyrus in relapsing-remitting multiple sclerosis with somatosensory disorder. Eur J Radiol. 2019;118:200-206.
10. Bernardi N F, Darainy M, Ostry D J. Somatosensory Contribution to the Initial Stages of Human Motor Learning. J Neurosci. 2015;35:14316-14326.
11. Andrews-Hanna J R, Smallwood J, Spreng R N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29-52.
12. Niendam T A, Laird A R, Ray K L, et al. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241-268.
13. Raichle M E. The brain's default mode network. Annu Rev Neurosci. 2015;38:433-447.
14. Vincent J L, Kahn I, Snyder A Z, et al. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328-3342.
15. Courtney S M, Petit L, Maisog J M, et al. An area specialized for spatial working memory in human frontal cortex. Science. 1998;279:1347-1351.
16. Owen A M, Evans A C, Petrides M. Evidence for a two-stage model of spatial working memory processing within the lateral frontal cortex: a positron emission tomography study. Cereb Cortex. 1996;6:31-38.
17. Rowe J B, Toni I, Josephs O, et al. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656-1660.
Figures

Figure 1. Demographic and clinical characteristics of CSVD patients and controls. Abbreviations: CSVD: cerebral small vessel disease; CSVD-c: CSVD with CMBs; CSVD-n: CSVD without CMBs; χ2: chi-square test; a: ANCOVA test; MoCA: Montreal Cognitive Assessment; AVLT: sum of Rey Auditory Verbal Learning Test (N1-7); SDMT: Symbol Digit Modalities Test; SCWT: sum of Stroop Color-Word Test (Stroop 1-3); TMT: Trail-Making Test; TMT-A+B: sum of TMT-A and TMT-B; FD_Jenkinson: frame-wise displacement.

Figure 2. Clusters with significantly altered ALFF/fALFF values among groups (ANOVA and the least-significant difference (LSD) post hoc test with Gaussian random field (GRF) correction, voxel-level p<0.001, cluster-level p<0.05). (1) The red-yellow areas denote higher ALFF values in the CSVD-c group than in the (A) control groups or (B) CSVD-n group. (2) The blue areas denote lower fALFF values in the CSVD-c group than in the control group.
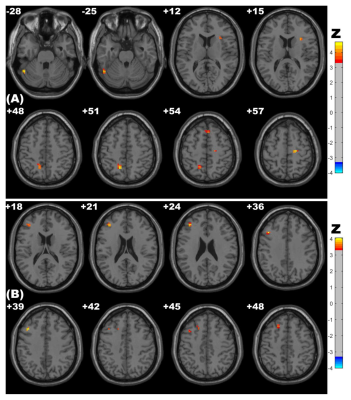
Figure 3. Clusters with significantly altered ReHo values among groups (ANOVA and LSD post hoc test with GRF correction, voxel-level p<0.001, cluster-level p<0.05). The red-yellow areas denote higher ReHo values in the CSVD-c group than in the (A) control groups or (B) CSVD-n group.

Figure 4. ROC curve for altered brain clusters that distinguish CSVD-c patients from controls. Abbreviations: ALFF: amplitude of low-frequency fluctuation; fALFF: fractional ALFF; ReHo: regional homogeneity; SMA: supplementary motor area; SFG: superior frontal gyrus.

Figure 5. Pearson's correlations among mean ALFF/fALFF/ReHo values of altered clusters and clinical parameters. Significance was set to p<0.05. Abbreviations: MoCA: Montreal Cognitive Assessment; AVLT: Auditory Verbal Learning Test; SDMT: Symbol Digit Modalities Test; TMT: Trail-Making Test; SCWT: Stroop Color-Word Test.