4590
Association between antiplatelet therapy and cerebral microbleeds determined by susceptibility weighted imaging in stroke-free pupolation1Department of Neurology, Beijing Tiantan Hospital, Capital Medical University; China National Clinical Research Center for Neurological Diseases, Beijing, China, 2Department of Neurology, The Third Hospital of Hebei Medical University, Shijiazhuang, Hebei, China, 3Department of Radiology, Beijing Geriatric Hospital, Beijing, China, 4Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China
Synopsis
In this cross-sectional, observational study, we investigated the association between antiplatelet therapy and cerebral microbleeds (CMBs) in community-based participants without stroke. The presence, count, and location of CMBs were evaluated by susceptibility weighted imaging. Antiplatelet therapy included aspirin use alone, clopidogrel use alone, cilostazol use alone, and dual antiplatelet therapy with aspirin and clopidogrel. We found that antiplatelet therapy was independently associated with CMBs at any location, particularly lobar CMBs, and aspirin use was a risk factor for any CMB and lobar CMBs. Our study suggests that optimizing antiplatelet treatment strategies in stroke-free population may decrease the risk of CMBs.
Introduction
Cerebral microbleeds (CMB) can predict the future risks of stroke, death, and dementia in the general population 1,2. It is beneficial to identify the risk factors for CMBs to facilitate the primary prevention of CMBs and subsequent intracerebral hemorrhage. Increasing evidence has shown that antiplatelet therapy may play a role in the occurrence of CMBs 3,4. However, there is still a debate on the association between antiplatelet therapy and CMBs 5,6, and few studies have focused on stroke-free populations. In addition, the imaging techniques are inhomogeneous for detecting CMBs in previous studies, and susceptibility-weighted imaging (SWI) is more sensitive to characterizing CMBs than T2*-weighted gradient echo imaging 7. This study aimed to investigate the association between antiplatelet therapy and CMBs utilizing SWI in the community-based participants without stroke.Methods
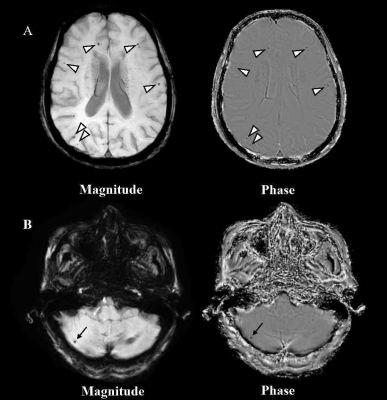
Study population: A total of 544 stroke-free participants (mean age 58.65 ± 13.66 years; 217 males) who participated in Cardio- and cerebrovascular Accident Monitoring, Epidemiology, and caRe quAlity system (CAMERA) study were included in this study. The Institutional Review Board approved this study, and all participants provided written informed consent before participating in the study. MR imaging: All participants underwent brain MRI on a 3T MR scanner (Philips Achieva TX, Philips Healthcare, Best, The Netherlands) using a custom-designed 36-channel neurovascular coil. For CMBs detection, we used the following imaging protocol and parameters: SWI: fast field echo sequence; repetition time 24 ms; echo times 5 ms, 10 ms, 15 ms, 20 ms; flip angle 17°; field of view 25.6 × 19.2 × 12.8 cm3; slice thickness 2 mm; in-plane resolution 0.6 × 0.6 mm2; and scan time 4 minutes 2 seconds. Imaging analysis: Two observers with > 3 years' experience in neuroimaging blinded to the clinical information reviewed the SWI images using a Digital Imaging and Communications in Medicine viewer (RadiAnt DICOM Viewer, Medixant, Poznan, Poland) with consensus. The presence, count, and location of CMBs were evaluated. The CMBs were classified into three types according to different locations: lobar CMBs, deep brain or infratentorial CMBs, and mixed CMBs (in both lobar and deep brain or infratentorial regions) (Figure 1). Statistical analysis: Continuous data were compared using the independent t-test (normal distribution) or Wilcoxon test (non-normal distribution). Categorical data were compared using the Chi-square test or Fisher exact test (if ≤20% of the expected cell counts were <5). The association between antiplatelet medication and the presence of any CMB was analyzed using multivariable logistic regression, the associations between antiplatelet medication and CMBs by location were analyzed using multinomial logistic regression, with no CMB at any location as reference for the response variable. Age, sex, history of smoke, hypertension, AF, antihypertensive agent usage, lipid-lowering agent usage, and the levels of HbA1c, LDL-C were adjusted as potential confounders. SAS version 9.4 software (SAS Institute Inc., Cary, NC, USA) was used to analyze all data. A two-sided P value < 0.05 was considered as statistical significance.Results
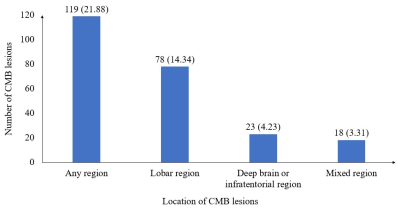
Of the 544 participants, 119 (21.88%) had at least one CMB, the prevalence of CMBs by location is shown in Figure 2. Of the 544 participants, 64 (11.76%) received antiplatelet therapy, of whom 44 (68.75%) used aspirin alone, six (9.38%) used clopidogrel alone, two (3.12%) used cilostazol alone, and 12 (18.75%) used dual antiplatelet therapy with aspirin and clopidogrel. Among the 64 antiplatelet drug users, 32 (50%) had CMBs, including 24 (37.5%) participants with lobar CMBs, four (6.25%) with deep or infratentorial CMBs, and four (6.25%) with mixed CMBs. The multivariable logistic regression analysis revealed that antiplatelet therapy was significantly associated with the presence of CMB at any location after adjustment (OR, 2.39; 95% CI, 1.25–4.59, P =0.009, Table 1). Considering CMB locations, antiplatelet therapy was significantly associated with lobar CMBs (OR, 2.89; 95% CI, 1.40–5.97, P = 0.004). Regarding the type of antiplatelet therapy, the use of aspirin was significantly associated with CMBs at any location (OR, 3.18; 95% CI, 1.50–6.72, P = 0.003) and lobar CMBs (OR, 3.71; 95% CI, 1.63–8.47, P = 0.018, Table 2). In the 64 antiplatelet drug users, participants with CMBs were predominantly males (71.88% vs 37.50%, P = 0.006), and had lower HDL-C level than those without CMB (1.37 ± 0.35 mmol/L vs 1.56 ± 0.35 mmol/L, P = 0.035).Discussion and Conclusion
Our study suggested that antiplatelet therapy was independently associated with CMBs at any location in a community-based stroke-free population. As for CMB location, we found that antiplatelet agents use was associated with lobar CMBs but not with deep or infratentorial CMBs. Among specific antiplatelet therapy, aspirin use is associated with any CMB and lobar CMBs. Our findings suggest that proper antiplatelet therapy in the primary prevention should also be considered based on its potential benefit and risk.Acknowledgements
We thank all the participants of the CAMERA (Cardio- and cerebrovascular Accident Monitoring, Epidemiology, and caRe quAlity system) study for their contribution.References
1.Akoudad S, Portegies ML, Koudstaal PJ, et al. Cerebral Microbleeds Are Associated With an Increased Risk of Stroke: The Rotterdam Study. Circulation. 2015;132(6):509-16.
2.Ding J, Sigurðsson S, Jónsson PV, et al. Space and location of cerebral microbleeds, cognitive decline, and dementia in the community. Neurology. 2017;88(22):2089-97.
3.Qiu J, Ye H, Wang J, et al. Antiplatelet Therapy, Cerebral Microbleeds, and Intracerebral Hemorrhage: A Meta-Analysis. Stroke. 2018;49(7):1751-4.
4.Liu S, Li C. Antiplatelet Drug Use and Cerebral Microbleeds: A Meta-analysis of Published Studies. J Stroke Cerebrovasc Dis. 2015;24(10):2236-44.
5.Kim CK, Kwon HT, Kwon HM. No significant association of aspirin use with cerebral microbleeds in the asymptomatic elderly. J Neurol Sci. 2012(1-2);319:56-8.
6.Graff-Radford J, Lesnick T, Rabinstein AA, et al. Cerebral Microbleeds: Relationship to Antithrombotic Medications. Stroke. 2021;52(7):2347-55.
7.Greenberg SM, Vernooij MW, Cordonnier C, et al. Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8(2):165-74.
Figures
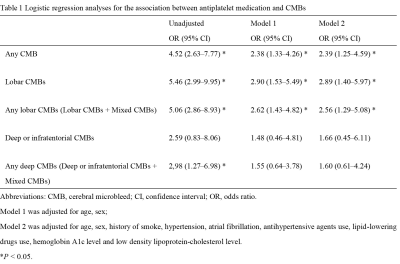
Logistic regression analyses for the association between antiplatelet therapy and CMBs
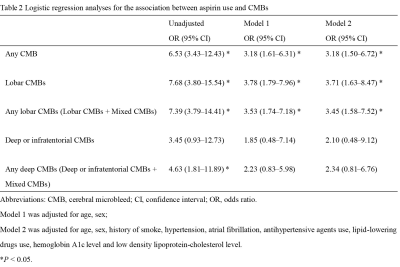
Logistic regression analyses for the association between aspirin use and CMBs