4589
Imaing the brain microstructures in patients of severe enlarged perivasclar spaces in the basal ganglia region using diffusion kurtosis imaging
Yuelei Lyu1, Shuna Yang1, Chen Zhang2, Qinglei Shi2, Guang Yang3, Wenli Hu1, and Qi Yang1
1Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China, 2MR Scientific Marketing, Siemens Healthineers, Beijing, China, 3Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai, China
1Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China, 2MR Scientific Marketing, Siemens Healthineers, Beijing, China, 3Shanghai Key Laboratory of Magnetic Resonance, School of Physics and Electronic Science, East China Normal University, Shanghai, China
Synopsis
This study used DKI to investigate possible microstructural alterations of brain tissues associated with plenty of EPVS in the basal ganglia (BG) in patients of SVD. Our preliminary results revealed that patients with EPVS in the BG showed significant reductions in kurtosis parameters from DKI and elevated diffusion parameters derived from DKI in bilateral putamen. We also found abnormal diffusion parameters in mainly bilateral BG, limbic system, temporal lobe,and other scatter brain regions. Our data suggests patients with plenty of EPVS in the BG might undergo microstructure impairments in the regions above, especially bilateral insular and BG.
Purpose
Perivascular spaces (PVS) become visible on magnetic resonance imaging (MRI) when enlarged. PVS are considered to play a role in normal brain homeostasis. Enlarged PVS (EPVS), a feature of several diseases, is related to small vessel disease(SVD). However, it is not clear how the PVS of SVD patients become widened. Diffusion tensor imaging (DTI) is one of the most commonly used methods in MRI, which can quantitatively measure microstructural integrity and organization in vivo. Diffusional kurtosis imaging (DKI), a natural extension of the DTI model, can acquire both diffusion metrics and kurtosis parameters simultaneously. DKI has exhibited improved sensitivity and specificity in assessing pathological changes in neural tissues compared to conventional DTI recently2.We aimed to explore microstructural impairments associated with plenty of EPVS in the basal ganglia region (BG) in patients of SVD using DKI method.
Methods
13 patients with plenty of EPVS in basal ganglia and 23 healthy controls (HCs) with age and sex matched were prospectively recruited in the study. All participants have written informed consent before each examination. Kurtosis parameters including kurtosis fractional anisotropy (FA), mean kurtosis (MK), axial kurtosis (AK), and radial kurtosis (RK) as well as diffusion metrics including mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD), derived from DKI were obtained. Voxel-based group differences in these metrics were compared using tract-based spatial statistics. All DKI data were calculated using the software (NeuDiLab) developed in-house based on the open resource tool DIPY (Diffusion Imaging In Python, http://nipy.org/dipy), and the parameter maps, FA, MK, AK, RK, MD, AD, and RD, were obtained. The DKI maps of all subjects were statistically analyzed using the two-sample t-test of in SPM12. P < 0.01 (Alphasim correction) and cluster size ≥5 were statistically significant. Then reports of brain regions with significance were generated.All data were collected on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthineers, Erlangen, Germany) through a 64-element head coil array. Regular T2-weighted fast-spin-echo images were obtained before acquiring diffusion kurtosis images. DKI was acquired by q-space Cartesian grid sampling with a radial grid size of 4 and half coverage using a twice-refocused SE EPI sequence and multiple q values with the following parameters: TE = 80 ms, TR =4200ms, slice thickness = 2.0 mm without a gap; matrix = 200 × 200; FOV= 200 × 200 mm2, the maximal b value = 3000 s/mm2, TA= 9.5 minutes, 70 axial slices covering the whole brain. We also used high-resolution anatomical imaging as an anatomical comparison, using a 5-min T1-weighted magnetization-prepared rapid gradient echo with the following parameters: TE = 2.27 ms, TR = 2300 ms, 208 slices, flip angle = 8, FOV= 256 × 256 mm2, voxel size = 1 mm isotropic.
Results
Compared to the HCs with age and gender matched, the patients with plenty of EPVS in the BG had significantly (P <0.001) decreased DKI-derived MK and RK in bilateral putamen (Figure 1, Figure 2, Table 1). However, no regions showed significantly change in AK. Compared to the HCs, the patients demonstrated significantly (P < 0.001) decrease of DKI-derived FA in multiple regions, slightly different with MK and RK: left superior parietal lobule, and white matter of superior frontal gyrus; right insula, angular gyrus and white matter of medial superior frontal gyrus; bilateral precuneus, postcentral gyrus ( Figure 3, Table 2). In comparison with HCs, the patients with plenty of EPVS in the BG exhibited significant increase of MD, AD and RD in similar regions: bilateral insula, basal ganglia nucleus (putamen, caudate, claustrum), limbic lobe (thalamus, hippocampus, parahippocampal gyrus and anterior cingulate), temporal lobe (transverse temporal gyrus, superior and middle temporal gyrus, left temporal pole), frontal lobe (superior, middle and medial frontal gyrus), white matter like internal capsule, superior regions of cerebellum and vermis and midbrain, right supra marginal gyrus, right middle and inferior occipital gyrus ( Figure 4, Figure 5, Table 3).Discussion
In this study, we used voxel-wise group analysis of DKI data to investigate possible microstructural alterations of brain tissues associated with plenty of EPVS in the BG in patients of SVD. Our preliminary results revealed that compared to HCs, patients with EPVS in the BG showed significant reductions in kurtosis parameters(MK and RK) from DKI and elevated diffusion parameters(MD,AD and RD) derived from DKI in bilateral putamen. We also found abnormal diffusion parameters(FA, MD,AD and RD) in the insula with decreased FA in peri-central sulcus regions and increased diffusion parameters in mainly bilateral BG, limbic system, temporal lobe, and other scatter brain regions. Our data suggests patients with plenty of EPVS in the BG might undergo microstructural impairments in above-mentioned areas, especially bilateral insular and BG.PVS form a network of spaces around cerebral microvessels that act as a conduit for fluid transport, exchange between cerebrospinal fluid and interstitial fluid1. DKI is reported to be more comprehensive than DTI in describing the complex water diffusion process in vivo3. Therefore, this study might be helpful to make a better understanding of the widening process of PVS.
Conclusion
In conclusion, DKI might be a useful tool to explore microstructural impairments associated with plenty of EPVS in the basal ganglia region in patients of SVD.Acknowledgements
We sincerely thank the participants in this study.References
- Brown R, Benveniste H, Black SE, et al. Understanding the role of the perivascular space in cerebral small vessel disease.[J] .Cardiovasc Res, 2018, 114: 1462-1473.
- Wang H, Wen H, Li J, et al. Characterization of Brain Microstructural Abnormalities in High Myopia Patients: A Preliminary Diffusion Kurtosis Imaging Study. Korean J Radiol. 2021 Jul;22(7):1142-1151
- Wu EX, Cheung MM. MR diffusion kurtosis imaging for neural tissue characterization.[J] .NMR Biomed, 2010, 23: 836-48.
Figures
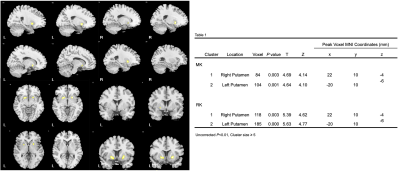
Figure 1 (Left) and Table 2 (Right). Left figure shows that DKI-derived MK in patients with EPVS decreased compared with healthy controls.(P < 0.001, cluster size ≥5 ); Table 2 shows the related voxels and peak MNI coordinates related to MK and RK.
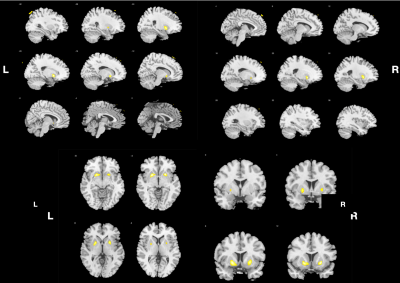
Figure 2 shows that DKI-derived RK in patients with EPVS decreased compared with healthy controls (P < 0.001, cluster size ≥5 ).
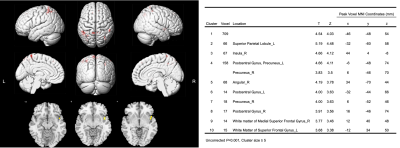
Figure 3 (Left) and Table 2 (Right). Left figure demonstrates that DKI-derived FA in patients with EPVS decreased compared with healthy controls.(P < 0.001, cluster size ≥5 ); Table 2 shows the related voxels and peak MNI coordinates.
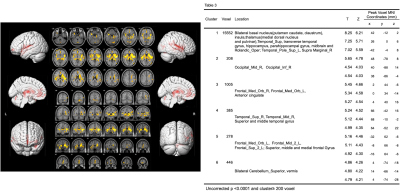
Figure 4 illustrates that DKI-derived MD in patients with EPVS decreased compared with healthy controls (P < 0.0001, cluster size ≥200 ).
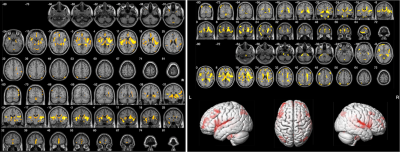
Figure 5 reveals that DKI-derived AD (left) and RD (right) in patients with EPVS decreased compared with healthy controls (P< 0.0001, cluster size ≥20 ).
DOI: https://doi.org/10.58530/2022/4589