4577
Does arterial spin labeling provide insights into long-term effects of radiation therapy in the healthy brain?1Neuroradiology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 2Pediatric Hematology, Oncology & Hemostaseology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 3Neurology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 4Center for Thrombosis and Hemostasis, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 5Institute of Medical Biostatistics, Epidemiology and Informatics, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 6Diagnostic and Interventional Radiology, University Hospital Frankfurt, Frankfurt, Germany, 7Otolaryngology, Head and Neck Surgery, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 8Diagnostic and Interventional Radiology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany, 9Radiation Oncology, University Medical Center of the Johannes Gutenberg University Mainz, Mainz, Germany
Synopsis
28 patients after earlier (1-45 years) whole brain radiation and 22 healthy controls were examined by arterial spin labeling to discover insights ASL could give in long-term effects of radiation therapy in the healthy brain. Relative cerebral blood flow and relative bolus arrival time showed increased values compared to the healthy control, which could be a sign for increased permeability and thickening of the vessels, two well described effects of radiation therapy ASL gives insight into.
Introduction
The number of long time survivors after radiation therapy (RT) of the brain is increasing [1]. Therefore, new methods to follow-up long term sequelae of RT in vivo are required. We wanted to identify the potential of arterial spin labeling (ASL) in the assessment of microvascular changes after RT of the brain. Therefore, we determined the differenced in bolus arrival time and cerebral blood flow of healthy and treated subjects with focus on the hippocampus, which is known to be very sensitive to radiation [2-4], the auditory cortex (AC), as patients get hearing impaired frequently [5-7] and the visual cortex as a region for comparison.Methods
28 patients aged 10 to 55 years (mean 25 years), who underwent whole brain RT were included into the study. Age at the time point of diagnosis was 4 to 17 years (mean 10 years) and the time since RT at the time point of ASL-MRI was 1 to 45 years (mean 15 years). A healthy control group (n=22, age 22 to 63 years, mean 32 years) was examined for comparison.All patients and volunteers underwent a pulsed ASL examination of the brain. Image processing was done using FSL [8-13] including motion correction and spatial normalization to MNI-space and an in house Python-script. Perfusion parameters were determined following the white paper recommendations [8] including partial-volume-correction [9, 10]. Cerebral blood flow (CBF) and bolus arrival time (BAT) were determined for the three regions and normalized by the mean values of the whole gray matter, further referred as relative CBF (relCBF) and relative BAT (relBAT).
For further evaluation, relCBF and relBAT were compared to the administered radiation dose and audiometric results.
Results
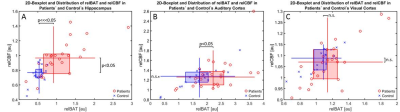
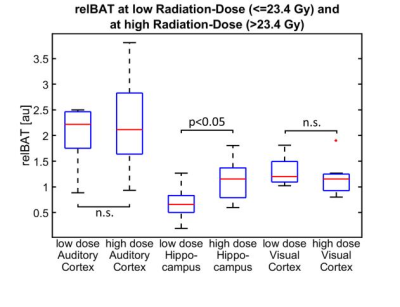
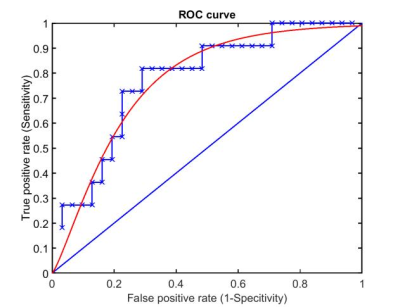
In the hippocampus, relCBF and relBAT showed significant higher values in the patient group than in the healthy controls. In the AC, only relBAT was significantly prolonged and in the visual cortex no significant differences could be found (Fig. 1). When compared to the administered radiation dose, significantly higher relCBF-values could be found for high radiation doses in the hippocampus (Fig. 2). A receiver operating curve (ROC)-analysis of relBAT and auditory threshold provides a positive predictive value of 71% and a sensitivity of 82% for hearing impairment for relBAT-values above 2.1 (Fig. 3).Discussion
Various reviews describe microvascular changes after RT. The changes occur predominantly in a rarefaction and thickening of the vessels and also a rise in permeability [4, 11-13]. Lee et al. show in their work using dynamic susceptibility contrast perfusion (DSC) imaging that a change in permeability can be observed in DSC-perfusion studies and therefore has to be a factor when performing perfusion studies in patients who underwent RT.ASL works by magnetically labeling blood-water prior to the examination area and measuring the amount of labeled water reaching the brain tissue by comparing it to an unlabeled image. As the amount of microspheres that get “stuck” in the capillary bed is a measure for CBF, the amount of labeled water that leaves the capillaries and enters the tissue is proportional to CBF [14]. An increase in permeability would alter the amount of arrested labeled molecules and mimic an increase in CBF [15]. Moreover, a thickening of the vessels would result in a higher volume to be filled by labeled blood which would in turn increase the BAT.
Taken together, our findings show, that the shift to the right of the patient group compared to the volunteer-group in Fig. 1A and 1B is caused by a dilatation of the microvasculature and the upshift in Figure 1A by an increased permeability. In the less affected visual cortex, shifts can be vaguely observed but are far from being significantly different.
When comparing the applied radiation doses to relBAT and relCBF, only in the region of hippocampus a significant difference between the low- and the high-dose group can be found (Fig. 2). This on the one hand reflects the higher radiation-sensitivity of the hippocampus (compared to other brain regions). On the other hand it may reflect a possible higher reactivity of the relBAT to changes in the tissue. Other perfusion studies have shown a higher sensitivity to perfusion changes of comparable parameters like mean-transit-time and time to drain compared to CBF [16].
The ROC-analysis of relBAT in the AC and the values of auditory threshold (with an impairment-threshold above 26 dB [17]) show that an increase of relBAT above 2.1 indicates a positive predictive value of 71% and a sensitivity of 82% for hearing impairment (Fig. 3). AC relBAT-values were depicted in a 2d box-plot (Fig. 1B), showing that relBAT-values of the healthy controls are predominant in the area below 2. This indicates that the damage caused by RT leads to impairments not only located in the cochlea [6] but also in the AC.
Conclusion
Our findings show that ASL can give insights into the microvascular changes after RT. A higher CBF mimics an increased permeability while a thickening of vessels is indicated by a prolonged BAT. Changes of relBAT in the AC, combined with the high prediction and sensitivity shown by the ROC-analysis, suggest an involvement of an AC-damage in case of a hearing impairment.Acknowledgements
No acknowledgement found.References
1. Deutsches Kinderkrebsregister, Jahresbericht 1997. 1997: Mainz, Germany.
2. Kazda, T., et al., Why and how to spare the hippocampus during brain radiotherapy: the developing role of hippocampal avoidance in cranial radiotherapy. Radiat Oncol, 2014. 9: p. 139.
3. Seibert, T.M., et al., Radiation Dose-Dependent Hippocampal Atrophy Detected With Longitudinal Volumetric Magnetic Resonance Imaging. Int J Radiat Oncol Biol Phys, 2017. 97(2): p. 263-269.
4. Warrington, J.P., et al., Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res, 2013. 50(6): p. 445-57.
5. Bass, J.K., et al., Hearing Loss in Patients Who Received Cranial Radiation Therapy for Childhood Cancer. J Clin Oncol, 2016. 34(11): p. 1248-55.
6. Bhandare, N., et al., Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys, 2010. 76(3 Suppl): p. S50-7.
7. Hua, C., et al., Hearing loss after radiotherapy for pediatric brain tumors: effect of cochlear dose. Int J Radiat Oncol Biol Phys, 2008. 72(3): p. 892-9.
8. Chappell, M.A., et al., Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magn Reson Med, 2011. 65(4): p. 1173-83.
9. Alsop, D.C., et al., Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med, 2015. 73(1): p. 102-16.
10. Chappell, M.A., et al., Variational Bayesian inference for a non-linear forward model. IEEE Transactions on Signal Processing, 2009. 57(1): p. 223-236.
11. Greene-Schloesser, D., et al., Radiation-induced brain injury: A review. Front Oncol, 2012. 2: p. 73.
12. Makale, M.T., et al., Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol, 2017. 13(1): p. 52-64.
13. Murphy, E.S., et al., Review of cranial radiotherapy-induced vasculopathy. J Neurooncol, 2015. 122(3): p. 421-9.
14. Wong, E.C., An introduction to ASL labeling techniques. J Magn Reson Imaging, 2014. 40(1): p. 1-10.
15. Parkes, L.M. and P.S. Tofts, Improved accuracy of human cerebral blood perfusion measurements using arterial spin labeling: accounting for capillary water permeability. Magn Reson Med, 2002. 48(1): p. 27-41.
16. Othman, A.E., et al., Volume perfusion CT imaging of cerebral vasospasm: diagnostic performance of different perfusion maps. Neuroradiology, 2016. 58(8): p. 787-92.
17. Organization", W.H., Report of the Informal Working Group on Prevention of Deafness and Hearing Impairment, Programme Planning. 1991: Geneva.
Figures